The Experience of Hematologic Oncology in Armenia
Abstract
This comprehensive nationwide overview of hematologic oncology is the first attempt in Armenia and aims to elucidate the key aspects of the field, encompassing epidemiological insights, diagnostic approaches, treatment strategies, patient outcomes, gaps and limitations. Although hematologic oncology in Armenia has made substantial progress in recent years, existing challenges and constraints require an integrated approach to ensure optimized patient care and achieve improved patient care.
Introduction
Armenia is a small, upper-middle-income country1. It has a population of 2.98 million people; 98.1% are Armenians2,3. Most of the population is urban (67.4%), with the largest predominance of residents concentrated in the capital Yerevan (almost 1.1 million people)2. Armenia has a decentralized healthcare system that is funded by general taxation. The system consists of three principal tiers: national or republican, regional, and municipal or community4,5. State health services offer complementary or subsidized healthcare, nevertheless, the basic benefits package is underfunded because of the low public budget for health, and the predominant share of healthcare expenses is borne by individuals through out-of-pocket payments, constituting a range from 78% to 85% of total health expenditures during the period 2017-20204,6. Since 2019, there has been a steady increase in coverage of services in Armenia, attributed to a rise in the health budget, and the country is making concerted efforts to attain universal health coverage, with a particular focus on expanding the basic benefits package4,6. The national health insurance program provides coverage for public health services, primary care, obstetric and postnatal care for all women and newborns, as well as medical services for special-status social groups such as people with special needs, children under 7, children under 18 without parental care, and military personnel. It also covers emergency resuscitation services, medical care for socially significant conditions like tuberculosis and HIV/AIDS, and partial coverage for other conditions, including cancer. Other sources of health financing in Armenia include voluntary health insurance and charitable foundations7,8.
This nationwide review is based on published national and international literature and expert opinion. Epidemiological data in the current review were collected from the registry of blood diseases at the Yeolyan Hematology and Oncology Center and the National Center of Oncology (NCO), two government-owned medical centers that diagnose and provide care to patients with hematological malignancies in our country. This article aims to offer a comprehensive account of the status of hematologic oncology in Armenia, encompassing aspects such as epidemiology, diagnosis, management, and advancements, as well as identifying challenges and future perspectives. The review will mainly focus on hematologic oncology in adults.
Discussion
Infrastructure and workforce
Hematological malignancies are mainly managed at Yeolyan Hematology and Oncology Center (formerly known as Hematology Center after Prof. R. H. Yeolyan), the only hematology center in Armenia. Lymphoma cases are rarely treated at the NCO. The NCO also provides radiation therapy. Both centers are located in the capital city of Yerevan and are publicly owned. The Yeolyan Center comprises several departments, including outpatient and inpatient adult hematology departments, pediatric cancer and blood disorders center, intensive care unit, stem cell transplantation department, department of surgery, blood bank, hemophilia and thrombosis center, pediatric palliative clinic, diagnostic laboratories, and psychological services. A team of 22 hematologists at the Yeolyan Center specializes in managing malignant and benign hematologic disorders. Additionally, 16 pediatric oncologists provide care for patients with hematologic disorders and solid tumors.
Over the last decade, several professional hematological and hematological/oncological associations have been established in Armenia. These organizations play an active role in organizing scientific meetings and collaborating with similar organizations from around the world, all aiming to foster the growth of specialists in this field in Armenia and promote scientific inquiry.
Diagnostic services
Yeolyan Center is the main referral center for the verification of blood disorders in the country. It has well-equipped morphological, biochemical, hemostasis, histological, microbiology, serology and immune hematology, molecular biology, and cytogenetics laboratories. Immunophenotyping and genetic analyses involving FISH, RT-PCR, and karyotyping are performed here9. We currently operate the only laboratory in Armenia offering immunophenotyping for leukemia diagnosis using an 8-color flow cytometer from Beckman Coulter. This capability allows us to provide detailed and accurate analyses essential for diagnosing and treating hematological malignancies. We prioritize a swift turnaround time for all samples and aim to deliver results within 24 hours. This ensures that patients and clinicians receive timely and actionable information. Performing these critical tests in-house not only accelerates the diagnostic process but also enhances quality control and result reliability. Genetic and molecular diagnostics face challenges like high testing costs, limited equipment availability, and inadequate insurance coverage. It is important to acknowledge that the scope of identified genetic abnormalities (such as FLT3, IDH, NPM1, etc.) is currently limited, impacting the ability to provide a comprehensive prognosis, risk stratification, and treatment decisions. However, ongoing initiatives aim to address these challenges. Laboratory facilities are being upgraded, and access to modern technologies, such as next-generation sequencing, is expanding. Consequently, there is a growing push for both government and private sector involvement to implement and subsidize these tests, making them more affordable for patients.
Improving molecular and genetic testing capabilities in Armenia has the potential to enhance patient outcomes. By enabling earlier and more precise diagnoses, these advancements allow for treatments to be more effectively tailored to individual patients, which can lead to more effective care and potentially better survival rates.
The Stem Cells Laboratory manages the processing and cryopreservation of stem cells from peripheral blood, bone marrow, and cord blood to ensure viability for transplantation. The laboratory has five cryostores, each capable of storing up to 100 bags. Samples undergo rigorous testing before processing, adhering to Good Manufacturing Practice standards in dedicated A and B-class laboratories according to GMP cleanroom quality standards. The laboratory features a biobank capable of preserving stem cells with uninterrupted biological activity over extended periods9. Histology and immunohistochemistry for lymphomas are also available at several private and public labs. Genetic analysis is also available at a few labs outside of Yeolyan Center. Radiology services are quite well developed in the country, and almost every large hospital has a CT, and there are a good number of MRIs as well in the country. Two PET-CTs are currently operating in Yerevan – a public one and a private one.
Treatment and outcome of hematologic neoplasms in Armenia
Acute leukemias
Acute leukemias (AL) are characterized by the clonal proliferation of malignant blast cells in the bone marrow along with impaired normal hematopoiesis. The two major subtypes of AL include acute myelogenous leukemia (AML) and acute lymphoblastic leukemia (ALL)10,11.
In Armenia, the age-adjusted incidence rates of AML and ALL from 2012 to 2018 were 1.9 and 1.5 per 100,000 population, respectively. Rates are similar to those in low- or middle-income countries; however, they are lower than in developed countries12. For instance, in the US, the annual rate of new cases of AML and ALL was 4.1 and 1.8 per 100 000 people, respectively13,14. The risk stratification for AML is based on European LeukemiaNet (ELN) classification15 with some limitations. In ALL, risk is assessed using age, white blood cell count, cytogenetics, molecular markers, minimal residual disease (MRD) levels, and response to initial therapy.
In Armenia, the main induction chemotherapy regimen for newly diagnosed AML patients is “7+3”16. Consolidation chemotherapy is mainly performed with high-dose cytarabine (HiDAC), although some patients may receive consolidation with “7+3” followed by maintenance therapy with cytarabine and 6-mercaptopurine17. For patients who are ineligible for intensive chemotherapy, lower-intensity therapies include hypomethylating agents (usually azacytidine), low-dose cytarabine (LDAC) +/- venetoclax18,19,20.
Therapy for acute promyelocytic leukemia typically involves regimens that incorporate all-trans-retinoic acid (ATRA) and intravenous arsenic trioxide (ATO)21, and additional anthracyclines for high-risk patients22.
In Armenia, the primary treatment regimen for adult patients with ALL under 55 years old was the GMALL 07/2003 protocol, replaced by GMALL 2017 version 3 in 202123,24. Patients aged 55 years and older are treated with the GMALL 2017 protocol for patients over 55 years of age or the GMALL 1989 protocol25. Patients with Philadelphia chromosome-positive ALL (Ph+ ALL) are treated with the GMALL 09/2017 protocol for Ph+ ALL, which includes the BCR-ABL1 tyrosine kinase inhibitor (TKI) imatinib.
Unfavorable-risk acute leukemias and relapsed/refractory (RR) disease patients are being considered for undergoing allogeneic hematopoietic cell transplantation (HCT) abroad, with the associated costs covered by the patients themselves. In August 2023, the first allogeneic HCT in Armenia for a child with leukemia was performed, and this treatment modality will gradually be available to other patients, eliminating the need to go abroad for these procedures.
Myelodysplastic syndromes
Myelodysplastic syndromes (MDS), a heterogeneous group of hematologic neoplasms, have an annual incidence of approximately 4 per 100 000 people, according to the US SEER database26. Based on unpublished data of the retrospective analysis covering 2008-2020, the incidence of MDS in Armenia is around 10 times lower compared to the US, standing at 0.35 cases per 100 000 population, indicating a significant epidemiological deviation from international data, probably due to the population number. The median age at diagnosis of individuals with MDS in Armenia was 62 years (age range, 19-84). The male-to-female ratio revealed a slight male predominance, with a ratio of 1.2 males for every female, comparable with US data27. The distribution of MDS subtypes within the Armenian cohort reveals that MDS with multilineage dysplasia (MDS-MLD), MDS with single lineage dysplasia (MDS-SLD), and MDS with excess blasts type 2 (MDS-EB2) were the most prevalent forms, comprising 29.24%, 27.3%, and 12.3% of cases, respectively. In Armenia, the distribution across risk categories based on the IPSS-R was as follows: very low (41%), low (25%), intermediate (16%), high (9%), and very high (9%). One comparative analysis of MDS subtype distribution revealed approximately 17% very low, 40% low, 20% intermediate, 12% high, and 11% very high-risk MDS in Western countries. In contrast, Asian countries had a higher prevalence of high-risk subtypes, with the distribution being approximately 4% very low risk, 32% low risk, 29% intermediate risk, 18% high risk, and 17% very high risk28.
The predominant treatment is supportive care, including blood transfusions for 41.5% of patients, though iron chelation therapy is inconsistently applied. Depending on the availability, treatment with azacytidine (7.5%), erythropoiesis-stimulating agents (6.6%), lenalidomide (3%), and allogeneic HCT (1.8%) were performed in some patients. A cytogenetic analysis was initiated in 2021, enabling us to perform risk stratification using IPSS-R and IPSS. Before this, routine cytogenetic testing was not conducted, and survival calculations for risk groups were not performed. In the Armenian MDS cohort, the median progression-free survival was 57 months, while the median overall survival (OS) reached 73.3 months. Globally, patients with lower-risk MDS have a median OS of 3-10 years, while patients with higher-risk disease have a median survival of less than 3 years29.
Myeloproliferative neoplasms
Chronic myeloid leukemia (CML) is a myeloproliferative disorder that can be cured with TKIs with a 5-year OS rate of over 90%30. The incidence of CML in Armenia from 2014 to 2018 per 100 000 people was 0.7, 0.6, 0.8, 0.4, and 0.9, for each respective year. These rates resemble those observed in Europe and indicate an upward trend31. Treatment of CML in Armenia improved significantly with TKIs. Since 2003, frontline imatinib therapy has become a standard for all CML patients. Subsequently, in 2017, ponatinib and nilotinib were also available30. In 2014-2018, a total of 102 patients were diagnosed with CML (including 4 pediatric patients). The sex distribution exhibited a male-to-female ratio of 1.17:1. Initially, 85% of patients were diagnosed in the chronic phase, while 9% were classified as the accelerated phase, and 6% as blast crisis. Patients categorized as high risk amounted to 15.7% based on Eutos, 28.4% according to Sokal, and 23.5% as indicated by the Euro risk scores. By the end of the study period, more than 75% of CML patients achieved complete remission, approaching rates set by developed countries31. The 5-year OS rate for the period 2010-2015 stood at 92% which aligns with global statistics30. Data on disease-free survival for CML is not yet available, and investigations are ongoing. There are currently 296 CML patients on active treatment, of whom 226 are treated with imatinib, 45 with nilotinib, and 25 with ponatinib. Starting from January 2021, regular molecular monitoring and monitoring-based treatment have been conducted in Armenia, which opens the possibility of implementing in October 2022 a pilot program of treatment-free remission with regular molecular monitoring, currently including 3 patients.
Primary myelofibrosis (PMF), polycythemia vera (PV), and essential thrombocythemia (ET) are the main Philadelphia chromosome-negative myeloproliferative neoplasms (MPN), which is a heterogeneous group of clonal blood disorders frequently accompanied by mutations in JAK2, CALR, or MPL, although not consistently32. A retrospective study examining the pattern of MPN in Armenia in the 2005-2019 period reported the mean yearly incidence of MPN of 1.84 cases per 100 000 people, comprising 2.10 for males and 1.64 for females33. Incidence rates of patients with Ph-negative MPNs are presented in Figure 1 32. The PMF had the highest annual average incidence rate at 1.09 per 100 000 population in 2018, while PV recorded a rate of 0.89 in 2016. In contrast, ET had the lowest incidence rate, standing at 0.7 per 100 000 population in 201632. In comparison, the US rates were 1.55 for ET, 1.57 for PV, and 0.44 per 100,000 person-years for PMF, with ET showing an increasing trend34. Another retrospective study examined data from PMF patients from 2010 to 2020 in Armenia. The median age of patients was 60 years (range, 28-88), and 43% were female. Among 112 patients, only a small subset of patients had their JAK2 or MPL mutations, along with other clonal indicators, evaluated, 11 and 2 patients, respectively. CALR was not examined in any patient. Furthermore, 12 patients were evaluated for BCR/ABL and showed a negative result. Over 74% of patients were prescribed hydroxycarbamide, 5.3% underwent treatment with ruxolitinib, and 6.3% with interferon-alpha, the remaining patients were under the follow-up35. Additionally, the study reported a 5-year OS of 40%, and a median survival of patients of 44 months (95% CI: 30-58 months)35. While the median OS in the US was 3.6 years34.
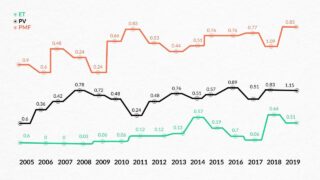
Figure 1. Incidence rates of patients with MPN depending on the type of disease during 2005-2019 (per 100 000 inhabitants) 33
A nationwide study on ET conducted in Armenia included individuals diagnosed with ET between 2003 and 2019. The median age at diagnosis was 52 years, and 68% of those diagnosed were female36. Notably, in the US the median age at PMF diagnosis was between 50 and 60 years, and there was no significant sex predominance37. Bone marrow biopsy confirmation for ET was available for over 77% of patients at the time of diagnosis. Genetic testing for the JAK2 V617F mutation was conducted on 13 patients (17%) during the study period. Regarding initial treatments, hydroxyurea was the primary choice (92%), followed by low-dose aspirin (6.4%) and interferon-alpha (1.6%) for a subset of patients. Analysis of outcomes revealed a 5-year OS of 87% for the studied group, which decreased to 70% by the 10-year mark36. For comparison, data from 7 international centers showed that the 10-year survival rate was 89%38. Data on PV is currently being collected, will be documented, and reported. The main treatment options include hydroxyurea, ruxolitinib, and phlebotomy.
Chronic lymphocytic leukemia
Chronic lymphocytic leukemia (CLL) stands as the most prevalent form of leukemia among adults. The integration of diverse targeted medications significantly improved the outcomes for CLL patients in the developed world39. The annual incidence of CLL in Armenia, calculated for the period 2020-2022, was 2.1 cases per 100 000 people. This is two times lower than the rate in the US, where the incidence is 4.6 per 100 000 population40. This substantial difference could be attributed to lower rates of insurance-covered preventive screenings, reduced clinic visits by patients with mild or no obvious clinical symptoms, and a lower average life expectancy of 75.55 years in 2023, compared to 79.11 in the US41,42). Upon conducting a retrospective study of all deceased CLL patients in Armenia spanning from 2016 to 2021, it was revealed that the median age of disease onset was 65 years, with a higher occurrence in males (with a male/female ratio of 1.4:1). The most prevalent stage at diagnosis, according to the Rai-Binet classification, was stage IIB43. Within the examined population, 69% of patients underwent treatment for CLL and the most used regimens were bendamustine + rituximab44, fludarabine + cyclophosphamide, or fludarabine + cyclophosphamide + rituximab45. Ongoing research aims to provide insights into the outcomes and long-term effects in patients diagnosed with CLL.
Multiple Myeloma
Multiple myeloma (MM) stands as the second most prevalent form of blood cancer in adults, resulting in approximately 117 000 fatalities each year worldwide46. Notably, modern progress in diagnostic methodologies and therapeutic alternatives for MM has revolutionized our understanding of the ailment, resulting in heightened survival rates and enhanced well-being among affected individuals47. Nonetheless, the advancement in management and comprehensive care in low- or middle-income countries lags, resulting in unfavorable patient outcomes48. A retrospective cohort study revealed that between 2006 and 2018 in Armenia, the yearly average incidence rate for MM was 1.2 per 100 000 people. Notably, a considerable rise was evident in 2018 when compared to 2006, with rates of 1.9 and 0.7 per 100 000 people, respectively. No discernible sex variations were found in the overall incidence of MM throughout the study period49. Globally, the incidence rate of MM is approximately 2 cases per 100 000 individuals, although this figure varies significantly. The highest rates are observed in highly developed countries like the US and Western Europe (≥4 per 100 000 population), probably due to greater awareness of the disease and more available diagnostic techniques50. Like other developing regions, Armenia experiences remarkably lower survival rates among MM patients compared to countries with more advanced economies mainly due to the availability of new targeted therapies in developed countries. A recent study conducted in Armenia revealed that the 1-year OS rates for MM patients diagnosed from 2008 to 2016 were 64.1% to 68.1%, and the 5-year OS was 17.6% to 18.5%49. In comparison, 5-year relative survival in the US was 59.8%51. Several crucial tools are unavailable in Armenia, primarily due to economic constraints. These tools encompass the FISH panel for MM, metaphase cytogenetics, gene expression profiling, and plasma cell labeling index48.
At the Yeolyan Center, the preference leans towards utilizing the VCD regimen (bortezomib, cyclophosphamide and dexamethasone)52 more frequently when compared to the VRD regimen (bortezomib, lenalidomide and dexamethasone)53, mainly due to the relatively elevated cost associated with lenalidomide. Among the less commonly employed regimens, we give preference to the lenalidomide and dexamethasone (Rd) protocol54, followed by cyclophosphamide and prednisolone (CP)55, bortezomib in conjunction with doxorubicin and dexamethasone (PAD)56, bortezomib combined with thalidomide and dexamethasone (VTD)57, or the vincristine, carmustine, melphalan, cyclophosphamide, and prednisone (VBMCP) regimen58, listed in order of frequency48. Patients under the age of 65 who meet the eligibility criteria undergo autologous stem cell transplantation (ASCT). Novel agents such as carfilzomib, daratumumab, and pomalidomide are typically reserved for second-line treatment strategies and patients rarely can afford them.
Additionally, the use of older regimens such as melphalan and prednisolone (MP)59, CP or VBMCP is still maintained, as their treatment costs are comparatively lower. Among the less frequently employed approaches, thalidomide and dexamethasone (TD)60 and the combination of cyclophosphamide, lenalidomide and dexamethasone (CRD) are also considered61.
Non-Hodgkin lymphoma
A concerning rise in the incidence of hematological malignancies has notably been exhibited in the escalating prevalence of non-Hodgkin lymphoma (NHL). As the most prevalent hematological malignancy worldwide, NHL accounts for nearly 3% of cancer diagnoses and fatalities62. A retrospective analysis of the amassed data revealed an average annual incidence of 4.34 NHL cases per 100 000 individuals during the 2017-2021 period in Armenia62. Comparative assessment with prior studies (1998-2004 and 1966-1971) unveiled a surge in NHL incidence rates, with a 1.5-fold and 4-fold increase, respectively. Notably, a substantial upswing occurred in 2019 compared to 2017, with rates of 5.9 and 3.3 NHL cases per 100 000 individuals, respectively. Age-standardized risk assessment demonstrated NHL rates of 4.8 among males and 3.9 among females. In both sexes, the elevated incidence was prominent in the age group of 55 years and older. This study illuminated a substantial escalation in NHL incidence rates across the examined period62. Ongoing endeavors are directed toward dissecting the statistics about specific lymphoma subtypes. Preliminary findings indicate that in Armenia diffuse large B-cell lymphoma (DLBCL), the most common type of aggressive NHL, accounts for 30% of cases similar to international data, while unclassified lymphomas constitute 20%-25%62,63).
In Armenia, the treatment landscape for aggressive NHLs primarily revolves around the widely used R-CHOP protocol (rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone)64. Indolent forms of NHL that require treatment have several options available. The primary treatment approach often involves chemotherapy with or without rituximab, utilizing regimens like R-CVP (cyclophosphamide, prednisolone, rituximab and vincristine)65, CVP (cyclophosphamide, prednisolone and vincristine)66, BR (bendamustine and rituximab), R-CHOP, and rituximab monotherapy. Radiotherapy as first-line treatment is not usually performed. In recent years, cladribine has become the standard treatment for hairy cell leukemia, with pentostatin not being accessible.
The utilization of rituximab represents the primary immunotherapy option for many types of NHL treatment in Armenia. Rituximab is accessible in Armenia, albeit with certain difficulties associated with the availability of the drug in only a few pharmacies in the country, and sometimes with the need for pre-ordering. Notably, other immunotherapy agents are not officially registered in the country. This absence of formal registration presents obstacles in terms of insurance coverage, resulting in the requirement for patients to acquire these treatments out-of-pocket. This financial aspect can pose significant challenges for patients, limiting their access to these potentially life-saving therapies.
Currently, data on the survival rates of NHLs is unavailable. Ongoing research is essential to provide insights into the prognosis and treatment efficacy for patients with this condition.
Hodgkin lymphoma
Hodgkin lymphoma (HL) is one of the most treatable malignant diseases, boasting a 5-year survival rate of over 80%67. The annual age-adjusted incidence rate in Armenia over 15 years (2000-2014) was 2.3 cases per 100 000 individuals68, which closely matches the corresponding US data of 2.5 cases per 100 00069.
Classical Hodgkin lymphoma (cHL) constitutes 95% of all HL cases70. In Armenia, between 2015 and 2020, a total of 212 patients were diagnosed with cHL, according to data recently presented at the Society of Hematologic Oncology 2023 Annual Meeting. The mean age of those affected is 45 years, with a range spanning from 20 to 83 years. There is a slightly higher male-to-female ratio of 1.09:1. Histological subtypes of cHL were distributed as follows: nodular sclerosis accounts for 54.8%, mixed cellularity for 35.3%, rich in lymphocytes for 8.49%, and depleted in lymphocytes for 1.41%. When newly diagnosed, 56% of patients were presented with advanced disease. The 5-year OS is 91%, comparable to that of the developed world69. Approximately 67% of patients underwent treatment with the ABVD protocol (doxorubicin, bleomycin, vinblastine and dacarbazine)71, while the remaining 33% received treatment with the escalated BEACOPP protocol (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisolone)72.
In June 2023 at the Yeolyan Center, the Immune Oncology Research Institute, initiated a phase 2 investigator-sponsored clinical trial (ClinicalTrials.gov: NCT05891821) to evaluate the safety and efficacy of single-agent balstilimab (PD-1 inhibitor) in patients with RR cHL or primary mediastinal B-cell lymphoma73.
Radiation therapy
Specialized radiotherapy services for hematological patients are available in two institutions in Yerevan and both are located at the NCO. The main provider of radiation oncology services in the country is the NCO, which covers more than 80% of cancer radiotherapy services in Armenia. The treatment costs for all patients in this public center are completely covered through a state-funded program launched in 2019.
The External Beam Radiotherapy (EBRT) Department of the NCO operates two EBRT complexes: one telecobalt device (Terabalt, 2006, Czech Republic) and one single energy (6MV) linear accelerator with multileaf collimators. The department is currently staffed with 7 radiation oncologists, 5 medical physicists, and 7 radiotherapy technologists.
Although cobalt-based 2D conventional radiotherapy is still in use in some situations (e.g., palliation, prophylactic cranial irradiation, etc.) because of a scarcity of technical resources, most patients with hematological malignancies are treated with linac-based 3D conformal radiotherapy. Involved site radiation therapy is the main option for lymphoma patients with CT-based computerized 3D treatment planning and the use of PET-CT image registration when available. Multi-disciplinary team discussions for all, including hematological patients before decision-making for radiotherapy are mandatory at NCO. However, in many cases, patients are seen by radiation oncologists only after completion of chemotherapy or other treatments, and radiation oncologists often are not involved in the initial decision-making process.
There is also a private institution operating a dual-energy linear accelerator staffed with 3 radiation oncologists where some patients are treated on a pay-per-service basis.
Hence, with 3 EBRT units in the country with about 3 million population, against the internationally recommended 10-12, lack of local training resources and infrastructures for core radiation oncology specialists and gaps in staffing, radiotherapy is the “bottleneck” in the continuum of comprehensive cancer services, which also influences the management of the hematological oncology patients74. In addition, such modalities as image-guided radiotherapy, volumetric arc therapy, stereotactic radiotherapy, total body irradiation, and radioimmunotherapy currently are not available in Armenia.
This, along with the high fragmentation of cancer care services, the lack of national standards and guidelines in the field, and an ineffective communication culture between specialists, largely explains the low utilization rates of radiotherapy in the treatment of hematological malignancies in Armenia (Table 1), particularly for lymphomas and myeloma.
Table 1. Radiotherapy utilization rates for hematological malignancies in Armenia, 2020
| Type of malignancy | Number of primary cases in 2020 | Should have been irradiated according to internationally accepted RTU levels (Optimal RTU, %) | Received radiotherapy in 2020 (Actual RTU, %) | Absolute difference | |||
|---|---|---|---|---|---|---|---|
| Leukemia | 183 | 7 (4%) | 6 (3%) | -1 | |||
| Myeloma and plasmacytoma | 57 | 26 (45%) | 3 (5%) | -23 | |||
| Lymphoma | 181 | 132 (73%) | 49 (27%) | -83 | |||
| Abbreviation: RTU, radiotherapy utilization rate | |||||||
Considering the current and growing needs for modern radiotherapy services, in 2021 NCO initiated a 3-year radiation oncology capacity building program with the construction of a new technological building and acquisition of two new high-energy linear accelerators. The implementation of the program will allow for fundamentally improving the situation with radiotherapy services in the country and cover the needs of the country’s population in state-of-art radiotherapy modalities.
Stem cell transplantation
Since its inception in 2017, the Armenian Bone Marrow Transplant program has achieved significant milestones. Beginning with ASCT for multiple myeloma in 2 patients in April 2017, the program has completed a total of 108 ASCTs as of August 9, 2023. Of these, 85 procedures were conducted in adults, spanning ages 19 to 67, while 23 were performed in pediatric patients aged 2 to 17.
For adult patients, the diagnostic spectrum encompasses a range of conditions, including RR HL and various subtypes of RR NHL such as DLBCL, follicular lymphoma, mantle cell lymphoma, primary central nervous system lymphoma, peripheral T-cell lymphoma, and MM. Meanwhile, pediatric patients underwent ASCT for diagnoses including RR HL, medulloblastoma, and Ewing’s sarcoma, as well as high-risk neuroblastoma.
Looking ahead, the program plans to expand by implementing allogeneic stem cell transplants for patients with hematologic malignancies in the near future. Notably, in July 2021, the Yeolyan Center marked a milestone by performing its first allogeneic hematopoietic stem cell transplant (HCT) on a pediatric patient with sickle cell disease9. In August 2023 the center conducted its first allogeneic HCT on a pediatric leukemia patient. The transplant department has seven beds. The wards are equipped with a high-efficiency particulate air (HEPA) filtration system, laminar air flow and positive pressure.
Palliative care
Until 2021, Armenia lacked facilities to provide palliative care for pediatric patients. Specifically, children with serious conditions would often pass away either at home or in intensive care units, without receiving adequate pain relief or symptom management, and frequently being separated from their families. With support from the City of Smile charity, Armenia’s first pediatric care clinic was established at the Yeolyan Center in September 2021. The clinic is designed for children up to 18 years old with malignant diseases. It can accommodate up to five patients and is equipped with amenities and essential medical equipment. The City of Smile Charitable Foundation ensures a steady supply of medications and provides four daily meals. Since the clinic’s establishment, 29 children have received treatment.
Children and their families receive support from a psychosocial team, including bereavement support groups. If the parents and/or the child choose to return home, we coordinate with the primary physician and maintain ongoing communication with the family to ensure effective care and continued support. Additionally, the palliative care team is actively involved in the care and symptom management of children undergoing treatment in the pediatric oncology and hematology departments as needed, facilitating a smoother transition to the palliative clinic. This year, pilot mobile palliative care services for children under 18 will be launched in Yerevan and four nearby regions. The program, funded by the Ministry of Health, will also include children who require palliative care for conditions other than cancer. In 2022, a large 30-bed well-equipped, modern adult palliative care clinic was established at NCO, which provides inpatient and outpatient palliative care services for oncology patients, including those with hematologic malignancies. Few specialists also provide outpatient palliative care services throughout the country. Currently, pain control and supportive care service is in the process of establishment at the Yeolyan Center. There are several small, private hospices also operating in Armenia, where patients mostly could receive end-of-life care.
Future perspectives
In addition to the above-mentioned initiatives, there are currently several efforts underway to enhance the field of hematologic oncology in Armenia. Nationwide electronic prospective registries (acute leukemias, CLL, MM) are being implemented to enable a more accurate and comprehensive situation analysis. Registries for Ph-negative MPNs and MDS are already running. The full integration of e-health systems at the Yeolyan Center is planned to be completed within the next 2-3 years.
With the establishment of disease-specific working groups and fostering collaboration among multidisciplinary teams, Armenia can effectively implement and adapt diagnostic and treatment approaches in hematologic oncology, improving patient care and outcomes. Experienced professionals are assigned to lead each working group, arranging regular meetings to review progress, share insights, conduct a thorough review of current scientific literature for evidence-based diagnostic and treatment strategies, and adapt standardized guidelines as needed.
One of the main challenges is the availability and accessibility of medications in the country. While for the essential medications, the main issue is the official registration of certain medications in Armenia and the assurance of their quality, for the novel therapies the main obstacle is the cost of those medications. Armenia plans to implement nationwide compulsory medical insurance, which is expected to address current challenges. Key drugs such as TKIs of three generations are funded by The Max Foundation. Recently, second-generation Bruton’s tyrosine kinase (BTK) inhibitors for CLL patients have also become accessible due to the Foundation’s support. In addition, as part of this collaboration, zanubrutinib, a new and promising drug, has become available to our patients. Several initiatives are ongoing for novel medications to bring clinical trials to the country and make investigational therapies accessible for Armenian patients.
The implementation of allogeneic HCT and CAR-T cell therapy in Armenia is emerging, offering potentially curative options for our patients. We are developing and strengthening partnerships with international centers and foundations, including Cure2Children Foundation and Moffitt Cancer Center. These collaborations are vital for knowledge exchange, training, and research, and will help to establish and enhance these advanced therapies in Armenia.
Furthermore, there is an increasing emphasis each year on the advancement of scientific research in Armenia. This emphasis is reflected in the heightened engagement of professionals in conducting and participating in clinical trials, attending international scientific events, and fostering the promising careers of young specialists. These initiatives strengthen Armenia’s global scientific presence and foster healthcare development and leadership. Armenian hematologists-oncologists are also striving to make contributions to global medical and scientific endeavors. In this context, it is worth mentioning the commencement of the blastic plasmacytoid dendritic cell neoplasm (BPDCN) international registry in 2022 (ClinicalTrials.gov: NCT05430971), initiated by the Immune Oncology Research Institute, which is the only similar initiative globally and currently 17 centers from 14 countries are already part of it75. Given the absence of a consensus on the optimal treatment for BPDCN and its rarity, international collaboration is crucial. This collaboration aims to collect data on BPDCN patients, establish a comprehensive patient database, investigate the disease’s characteristics, evaluate prognostic factors and outcomes, and develop prospective treatment recommendations.
Our center has recently joined a Phase 1b, multicenter, open-label clinical trial (ClinicalTrials.gov: NCT04953897) that evaluates the pharmacokinetics, safety, and tolerability of multiple doses of oral decitabine and cedazuridine in cancer patients with severe renal impairment as well as those with normal renal function serving as matched control subjects. This study enrolls adult patients with AML, MDS, or solid tumors who are eligible for treatment with these medications76.
To further advance our understanding and management of hematologic malignancies, we propose several key areas for future research. One of them is the clinical significance of the JAK2 (V617F) allele burden in Ph-negative MPNs. Understanding its potential influence on disease course and management could provide critical insights into optimizing treatment protocols and improving patient stratification.
Conclusion
Considerable progress has been observed in the field of hematological oncology in Armenia in recent years; however, many challenges still need to be addressed. Some diagnostic tools are either unavailable or not routinely performed in the country due to financial constraints, highlighting existing gaps and limitations. The lack of genetic and molecular data hampers our understanding of the pathophysiology of diseases within the Armenian population and restricts personalized treatment approaches. The disparities in incidences of different types of hematologic malignancies are attributed to disease awareness and accessibility to diagnostic techniques. Additionally, the availability of advanced treatment options remains limited, such as targeted therapies or immunotherapies, remains limited. The scarcity of treatment options highlights the need to expand access to medications for patients with hematologic malignancies in the country. Despite these challenges, the medical community in Armenia remains committed to providing the best possible care. Efforts are underway to address these gaps by advocating for the registration of therapeutic agents and exploring avenues for funding assistance. As the healthcare landscape evolves, we hope that increased accessibility and support will improve the treatment options and outcomes of individuals facing hematologic malignancies in Armenia.
Abbreviations
| Abbreviation | Explanation |
|---|---|
| ABVD | Doxorubicin, bleomycin, vinblastine and dacarbazine |
| AL | Acute leukemias |
| ALL | Acute lymphoblastic leukemia |
| AML | Acute myelogenous leukemia |
| ATRA | All-trans-retinoic acid |
| ATO | Arsenic trioxide |
| ASCT | Autologous stem cell transplantation |
| BEACOPP | Bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisolone |
| BPDCN | Blastic plasmacytoid dendritic cell neoplasm |
| BR | Bendamustine and rituximab |
| BTK | Bruton's tyrosine kinase |
| CML | Chronic myeloid leukemia |
| CI | Confidence interval |
| CLL | Chronic lymphocytic leukemia |
| CT | Computed tomography |
| CRD | Cyclophosphamide, lenalidomide and dexamethasone |
| CVP | Cyclophosphamide, vincristine and prednisolone |
| DLBCL | Diffuse large B-cell lymphoma |
| EBRT | External beam radiotherapy |
| ELN | European LeukemiaNet |
| ET | Essential thrombocythemia |
| FISH | Fluorescence in situ hybridization |
| GMP | Good Manufacturing Practice |
| HCT | Hematopoietic cell transplantation |
| HEPA filtration | High-efficiency particulate air filtration |
| HiDAC | High-dose cytarabine |
| HL | Hodgkin lymphoma |
| cHL | Classical Hodgkin lymphoma |
| HIV/AIDS | Human immunodeficiency virus / acquired immunodeficiency syndrome |
| IPSS | International Prognostic Scoring System |
| IPSS-R | Revised International Prognostic Scoring System |
| LDAC | Low-dose cytarabine |
| LMICs | Low or middle-income countries |
| MDS | Myelodysplastic syndromes |
| MDS-EB2 | Myelodysplastic syndromes with excess blasts type 2 |
| MDS-MLD | Myelodysplastic syndromes with multilineage dysplasia |
| MDS-SLD | Myelodysplastic syndromes with single lineage dysplasia |
| MM | Multiple myeloma |
| MP | Melphalan and prednisolone |
| MPN | Myeloproliferative neoplasms |
| MRD | Minimal residual disease |
| MRI | Magnetic resonance imaging |
| NHL | Non-Hodgkin lymphoma |
| NCO | National Center of Oncology |
| OS | Overall survival |
| PAD | Bortezomib, doxorubicin and dexamethasone |
| PET-CT | Positron emission tomography-computed tomography |
| PMF | Primary myelofibrosis |
| PV | Polycythemia vera |
| R-CHOP | Rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone |
| R-CVP | Rituximab, cyclophosphamide, vincristine and prednisolone |
| Rd | Lenalidomide and dexamethasone |
| RR | Relapsed / refractory |
| RT-PCR | Reverse transcription polymerase chain reaction |
| RTU | Radiotherapy utilization rate |
| SEER | Surveillance, epidemiology and end results program |
| TD | Thalidomide and dexamethasone |
| TKI | Tyrosine kinase inhibitor |
| VCD | Bortezomib, cyclophosphamide and dexamethasone |
| VRD | Bortezomib, lenalidomide and dexamethasone |
| VTD | Bortezomib, thalidomide and dexamethasone |
| VBMCP | Vincristine, carmustine, melphalan, cyclophosphamide and prednisone |
Competing Interests: The authors declare no competing financial interests.
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
License
© Author(s) 2024.
This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, and unrestricted adaptation and reuse, including for commercial purposes, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
To view a copy of this license, visit https://creativecommons.org/licenses/by/4.0/.
References
-
World Bank. World Bank Open Data: Data for Armenia, Upper middle income [Internet]. 2023 [cited 2023 Jul 29]; [dataset]. Available from: https://data.worldbank.org
-
Worldometer. Armenia population [Internet]. 2023 [cited 2023 Jul 29]; [screen]. Available from: https://www.worldometers.info/world-population/armenia-population/
-
Government of the Republic of Armenia. About Armenia – Demographics [Internet]. [cited 2023 Jul 29]; Available from: https://www.gov.am/en/demographics/
-
Tsaturyan S, Scarpetti G. Health systems in action: Armenia. Copenhagen: WHO Regional Office for Europe; 2022.
-
Richardson E. Armenia: health system review. Health Syst Transit. 2013;15(4):1-99.
-
World Bank. Out-of-pocket expenditure (% of current health expenditure) – Armenia [Internet]. 2023 [cited 2023 Aug 1]; Available from: https://data.worldbank.org/indicator/SH.XPD.OOPC.CH.ZS?locations=AM
-
Global CPS Business and Legal Consulting. Health care system [Internet]. In: Invest in Armenia. [cited 2023 Aug 1]. Available from: http://investinarmenia.am/en/health-care-system
-
Petrosyan V, Martirosyan H. Armenia. In: Voluntary health insurance in Europe: country experience [Internet]. European Observatory on Health Systems and Policies; 2016 [cited 2023 Jul 31]. Available from: https://iris.who.int/handle/10665/330359
-
Hematology Center After Prof. R. Yeolyan [Internet]. [cited 2023 Sep 2]. Available from: https://blood.am/eng
-
Saif A, Kazmi SFA, Naseem R, Shah H, Butt MO. Acute myeloid leukemia: is that all there is? Cureus. 2018;10(8):e3198.
-
Chennamadhavuni A, Iyengar V, Mukkamalla SKR, Shimanovsky A. Leukemia. 2023 Jan 17. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–.
-
Khondkaryan L, Andreasyan D, Hakobyan Y, et al. Incidence and Risk Factors of Acute Leukemias in Armenia: A Population-Based Study. Asian Pac J Cancer Prev. 2022 Nov 1;23(11):3869-3875.
-
National Institutes of Health. Surveillance, Epidemiology, and End Results (SEER) Program: Acute Myeloid Leukemia – Cancer Stat Facts [Internet]. [cited 2023 Sep 5]. Available from: https://seer.cancer.gov/statfacts/html/amyl.html
-
National Institutes of Health. Surveillance, Epidemiology, and End Results (SEER) Program: Acute Lymphocytic Leukemia – Cancer Stat Facts [Internet]. [cited 2023 Sep 7]. Available from: https://seer.cancer.gov/statfacts/html/alyl.html
-
Döhner H, Wei AH, Appelbaum FR, Craddock C, DiNardo CD, Dombret H, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022 Sep 22;140(12):1345–77.
-
Rowe JM. The “7+3” regimen in acute myeloid leukemia. Haematologica. 2022 Jan 1;107(1):3.
-
Oxford Haematology NSSG. Cytarabine (Ara-C) high dose [Internet]. [cited 2023 Sep 4]. Available from: https://nssg.oxford-haematology.org.uk/myeloid/protocols/ML-4-cytarabine-ara-c-3g-m2.pdf
-
Dutton D. Venetoclax and Cytarabine (low dose) acute myeloid leukaemia [Internet]. 2023;(1). Available from: https://www.clatterbridgecc.nhs.uk/application/files/3416/8198/0974/Venetoclax_and_Cytarabine_Low_Dose_Acute_Myeloid_Leukaemia_v1.0.pdf
-
Oxford Haematology NSSG. Azacitidine and venetoclax [Internet]. [cited 2023 Sep 4]. Available from: https://nssg.oxford-haematology.org.uk/myeloid/protocols/ML-84-azacitidine-and-venetoclax-covid-19.pdf
-
Acute myeloid leukemia [Internet]. 2007 Sep 24 [cited 2023 Sep 4]. Available from: https://hemonc.medicine.ufl.edu/files/2013/07/AML1.pdf
-
Lo-Coco F, Avvisati G, Vignetti M, et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med. 2013 Jul 11;369(2):111–21.
-
Iland HJ, Bradstock K, Supple SG, et al. All-trans-retinoic acid, idarubicin, and IV arsenic trioxide as initial therapy in acute promyelocytic leukemia (APML4). Blood. 2012 Aug 23;120(8):1570–80.
-
Hoelzer D, Gökbuget N. Multicentric therapy optimization study of acute lymphatic leukemia in adults and adolescents from 18 years (GMALL 07/2003): therapy optimization by evaluating minimal residual disease – short protocol [in German]. 2003 Jul 30.
-
Gökbuget N. Consensus recommendation for the treatment of acute lymphoblastic leukemia (ALL) and lymphoblastic lymphoma (LBL) in adults/adolescents (18–55 years). Version 3 [in German]. 2017 Oct 16.
-
Volkova MA. Clinical oncohematology [in Russian]. Moscow: Medicine; 2001.
-
Aster JC, Stone RM. Clinical manifestations, diagnosis, and classification of myelodysplastic syndromes (MDS). In: Post TW, editor. UpToDate [Internet]. Waltham (MA): UpToDate; 2023 [cited 2023 Aug 20]. Available from: https://www.uptodate.com/contents/clinical-manifestations-diagnosis-and-classification-of-myelodysplastic-syndromes-mds
-
Wang F, Ni J, Wu L, Wang Y, He B, Yu D. Gender disparity in the survival of patients with primary myelodysplastic syndrome. J Cancer. 2019 Jan 30;10(5):1325–32.
-
Jiang Y, Eveillard JR, Couturier MA, et al. Asian Population Is More Prone to Develop High-Risk Myelodysplastic Syndrome, Concordantly with Their Propensity to Exhibit High-Risk Cytogenetic Aberrations. Cancers. 2021 Jan 27;13(3):481.
-
Sekeres MA, Taylor J. Diagnosis and Treatment of Myelodysplastic Syndromes: A Review. JAMA. 2022 Sep 6;328(9):872.
-
Harutyunyan L, Voskanyan A, Meliksetyan K, Sahakyan L, Chakmanyan A, Sargsyan G, et al. CML-307: Assessment of 5-Year OS and Treatment Options in Newly Diagnosed Ph + CML Patients in the Republic of Armenia. Clin Lymphoma Myeloma Leuk. 2021 Sep 1;21:S333.
-
Harutyunyan L, Meliksetyan K, Ivanyan A, Hakobyan Y. CML-218: Primary Diagnosed CML Patient 5-Year Treatment Outcomes and Disease Incidence in an Armenian Population. Clin Lymphoma Myeloma Leuk. 2020 Sep 1;20:S237–8.
-
Gerds AT, Gotlib J, Ali H, et al. Myeloproliferative Neoplasms, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Cancer Netw JNCCN. 2022 Sep;20(9):1033–62.
-
Sahakyan L, Ter-Grigoryan A, Badikyan M, et al. Incidence in PH-negative myeloproliferative neoplasms in Armenia from 2005 to 2019. Hematol Transfus Cell Ther. 2020 Oct 1;42:19–20.
-
Verstovsek S, Yu J, Scherber RM, et al. Changes in the incidence and overall survival of patients with myeloproliferative neoplasms between 2002 and 2016 in the United States. Leuk Lymphoma. 2022 Feb 23;63(3):694–702.
-
Voskanyan A, Ter-Grigoryan A, Meliksetyan K, et al. MPN-270 Challenges of the Management of Primary Myelofibrosis in Armenia. Clin Lymphoma Myeloma Leuk. 2022 Oct;22 Suppl 2:S332.
-
Grigoryan H, Badikyan M, Hambardzumyan L, et al. MPN-200: Essential Thrombocythemia in Armenia: A Nationwide Study. Clin Lymphoma Myeloma Leuk. 2020 Sep 1;20:S333–4.
-
SEER Hematopoietic and Lymphoid Neoplasm Database. Essential thrombocythemia [Internet]. SEER; [cited 2023 Sep 8]. Available from: https://seer.cancer.gov/seertools/hemelymph/51f6cf58e3e27c3994bd53ff/
-
Barbui T, Thiele J, Passamonti F, et al. Survival and Disease Progression in Essential Thrombocythemia Are Significantly Influenced by Accurate Morphologic Diagnosis: An International Study. J Clin Oncol. 2011 Aug 10;29(23):3179–84.
-
Mukkamalla SKR, Taneja A, Malipeddi D, Master SR. Chronic lymphocytic leukemia. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 [cited 2023 Sep 5]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470433/
-
National Institutes of Health. Surveillance, Epidemiology, and End Results (SEER) Program: Chronic Lymphocytic Leukemia – Cancer Stat Facts [Internet]. [cited 2024 Jan 9]. Available from: https://seer.cancer.gov/statfacts/html/clyl.html
-
Macrotrends. Armenia life expectancy 1950–2023 [Internet]. [cited 2023 Sep 19]. Available from: https://www.macrotrends.net/countries/ARM/armenia/life-expectancy
-
Macrotrends. U.S. life expectancy 1950–2023 [Internet]. [cited 2023 Sep 19]. Available from: https://www.macrotrends.net/countries/USA/united-states/life-expectancy
-
Gribben JG. How I treat CLL up front. Blood. 2010 Jan 14;115(2):187–97.
-
Oxford Haematology NSSG. Bendamustine + rituximab (BR) for CLL [Internet]. [cited 2023 Sep 8]. Available from: https://nssg.oxford-haematology.org.uk/lymphoma/documents/lymphoma-chemo-protocols/L-44-bendamustine-70-r-cll.pdf
-
Oxford Haematology NSSG. Fludarabine cyclophosphamide rituximab (FCR) intravenous [Internet]. [cited 2023 Sep 8]. Available from: https://nssg.oxford-haematology.org.uk/lymphoma/documents/lymphoma-chemo-protocols/L-29-flu-cy-r-iv.pdf
-
Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–49.
-
Kazandjian D. Multiple myeloma epidemiology and survival: A unique malignancy. Semin Oncol. 2016 Dec;43(6):676–81.
-
Oganesyan A, Ghahramanyan N, Mekinian A, Bejanyan N, Kazandjian D, Hakobyan Y. Managing multiple myeloma in a resource-limited region: Diagnosis and treatment in Armenia. Semin Oncol. 2021;48(4–6):269–78.
-
Ghazaryan N, Danelyan S, Bardakhchyan S, Saharyan A, Sahakyan L. Multiple myeloma in Armenia during the period 2006–2018: facts and discussion. BMC Cancer. 2021 Aug 21;21(1):941.
-
Mateos MV, Ailawadhi S, Costa LJ, et al. Global disparities in patients with multiple myeloma: a rapid evidence assessment. Blood Cancer J. 2023 Jul 18;13(1):1–9.
-
National Institutes of Health. Surveillance, Epidemiology, and End Results (SEER) Program: Myeloma – Cancer Stat Facts [Internet]. [cited 2023 Sep 7]. Available from: https://seer.cancer.gov/statfacts/html/mulmy.html
-
SWAG Cancer Alliance. Bortezomib, cyclophosphamide and dexamethasone (VCD) [Internet]. [cited 2023 Sep 4]. Available from: https://www.swagcanceralliance.nhs.uk/wp-content/uploads/2020/10/VCD.pdf
-
Oxford Haematology NSSG. Bortezomib lenalidomide and dexamethasone (VRD) [Internet]. [cited 2023 Sep 4]. Available from: https://nssg.oxford-haematology.org.uk/myeloma/pdf-protocols/MM-6-bortezomib-len-dex.pdf
-
SWAG Cancer Alliance. Lenalidomide and dexamethasone (Rd) – second line onwards [Internet]. [cited 2023 Sep 4]. Available from: https://www.swagcanceralliance.nhs.uk/wp-content/uploads/2020/10/Lenalidomide-and-dex-subsequent.pdf
-
Oxford Haematology NSSG. Oral cyclophosphamide with or without prednisolone [Internet]. [cited 2023 Sep 4]. Available from: https://nssg.oxford-haematology.org.uk/myeloma/pdf-protocols/MM-13-cyclophosphamide.pdf
-
University Hospital Southampton NHS Foundation Trust. PAD (IV) – bortezomib (IV), dexamethasone, doxorubicin [Internet]. [cited 2023 Sep 4]. Available from: https://www.uhs.nhs.uk/Media/UHS-website-2019/Docs/Chemotherapy-SOPs1/Myeloma/MyelomaPAD-BortezomibIVDexamethasoneDoxorubicinVer1.pdf
-
Oxford Haematology NSSG. Bortezomib thalidomide and dexamethasone (VTD21), 21-day cycle [Internet]. [cited 2023 Sep 4]. Available from: https://nssg.oxford-haematology.org.uk/myeloma/pdf-protocols/MM-7-bortezomib-thal-dex-21d.pdf
-
Kyle RA, Jacobus S, Friedenberg WR, Slabber CF, Rajkumar SV, Greipp PR. The treatment of multiple myeloma using vincristine, carmustine, melphalan, cyclophosphamide, and prednisone (VBMCP) alternating with high-dose cyclophosphamide and α2β interferon versus VBMCP. Cancer. 2009;115(10):2155–64.
-
Oxford Haematology NSSG. Oral melphalan ± prednisolone [Internet]. [cited 2023 Sep 4]. Available from: https://nssg.oxford-haematology.org.uk/myeloma/pdf-protocols/MM-20-melphalan-pred.pdf
-
Oxford Haematology NSSG. Thalidomide with or without dexamethasone [Internet]. [cited 2023 Sep 4]. Available from: https://nssg.oxford-haematology.org.uk/myeloma/pdf-protocols/MM-23-thalidomide-plus-minus-dex.pdf
-
Oxford Haematology NSSG. Lenalidomide, cyclophosphamide and weekly dexamethasone [Internet]. [cited 2023 Sep 4]. Available from: https://nssg.oxford-haematology.org.uk/myeloma/pdf-protocols/MM-17-lena-cyclop-dex.pdf
-
Sahakyan L, Harutyunyan L, Voskanyan A, et al. ABCL-258 Incidence Patterns of Non-Hodgkin Lymphoma in the Republic of Armenia. Clin Lymphoma Myeloma Leuk. 2022 Oct 1;22:S366.
-
Padala SA, Kallam A. Diffuse large B-cell lymphoma. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 [cited 2023 Sep 8]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK557833/
-
University Hospital Southampton NHS Foundation Trust. RCVP – cyclophosphamide, prednisolone, rituximab, vincristine (Ver. 1.2) [Internet]. [cited 2023 Sep 4]. Available from: https://www.uhs.nhs.uk/Media/UHS-website-2019/Docs/Chemotherapy-SOPs1/Lymphoma/RCVP-Cyclophosphamide-Prednisolone-Rituximab-Vincristine-Ver-1.2.pdf
-
University Hospital Southampton NHS Foundation Trust. RCVP – cyclophosphamide, prednisolone, rituximab, vincristine (Ver. 1.2) [Internet]. [cited 2023 Sep 4]. Available from: https://www.uhs.nhs.uk/Media/UHS-website-2019/Docs/Chemotherapy-SOPs1/Lymphoma/RCVP-Cyclophosphamide-Prednisolone-Rituximab-Vincristine-Ver-1.2.pdf
-
University Hospital Southampton NHS Foundation Trust. CVP – cyclophosphamide, prednisolone, vincristine (Ver. 1.2) [Internet]. [cited 2023 Sep 4]. Available from: https://www.uhs.nhs.uk/Media/UHS-website-2019/Docs/Chemotherapy-SOPs1/Lymphoma/CVP-Cyclophosphamide-Prednisolone-Vincristine-Ver-1.2.pdf
-
Bhurani D, Nair R, Rajappa S, et al. Real-World Outcomes of Hodgkin Lymphoma: A Multi-Centric Registry From India. Front Oncol. 2021;11:799948.
-
Arakelyan J, Movsisyan A, Sargsyan L, et al. Incidence patterns and review of Hodgkin lymphoma in the Republic of Armenia. Ecancermedicalscience. 2021 Nov 18;15:1319.
-
National Institutes of Health. Surveillance, Epidemiology, and End Results (SEER) Program: Hodgkin lymphoma – Cancer Stat Facts [Internet]. [cited 2023 Sep 7]. Available from: https://seer.cancer.gov/statfacts/html/hodg.html
-
Kaseb H, Babiker HM. Hodgkin lymphoma. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 [cited 2023 Aug 4]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK499969/
-
Oxford Haematology NSSG. ABVD [Internet]. [cited 2023 Sep 4]. Available from: https://nssg.oxford-haematology.org.uk/lymphoma/documents/lymphoma-chemo-protocols/L-8%20-%20abvd.pdf
-
Oxford Haematology NSSG. BEACOPP-14 / BEACOPP-escalated [Internet]. [cited 2023 Sep 4]. Available from: https://nssg.oxford-haematology.org.uk/lymphoma/documents/lymphoma-chemo-protocols/L-18-beacopp-14-or-beacopp-escalated.pdf
-
Immune Oncology Research Institute. Assessment of the safety and efficacy of balstilimab for the treatment of relapsed/refractory lymphomas (IMMONC0001) [Internet]. clinicaltrials.gov; 2023 May [cited 2023 Sep 1]. Report No.: NCT05891821. Available from: https://clinicaltrials.gov/study/NCT05891821
-
Barton MB, Jacob S, Shafiq J, et al. Estimating the demand for radiotherapy from the evidence: A review of changes from 2003 to 2012. Radiother Oncol. 2014 Jul 1;112(1):140–4.
-
Immune Oncology Research Institute. Blastic plasmacytoid dendritic cell neoplasm (BPDCN) international registry [Internet]. clinicaltrials.gov; 2023 Aug [cited 2023 Sep 1]. Report No.: NCT05430971. Available from: https://clinicaltrials.gov/study/NCT05430971
-
Astex Pharmaceuticals, Inc. Study to evaluate the pharmacokinetics and safety of oral decitabine and cedazuridine in cancer patients with renal impairment [Internet]. clinicaltrials.gov; 2021 Jul 9 [cited 2024 Aug 31]. Report No.: NCT04953897. Available from: https://clinicaltrials.gov/study/NCT04953897





