Evaluation of Virus-specific T-lymphocytes in CD45RA-depleted Products before and after Long-term Cryopreservation: Impact of Cell Resting and Storage Duration
Abstract
Introduction: CMV, EBV, and ADV infections represent major complications following hematopoietic stem cell transplantation (HSCT). Adoptive immunotherapy using virus-specific T-lymphocytes (VSTs), which are present in the CD45RA-depleted fraction, is a promising alternative, yet accurate quantification of functional VSTs in donor products remains technically challenging.
Objective: To assess the detectability and functional activity of VSTs in CD45RA-depleted donor lymphocyte infusion (DLI) products before and after cryopreservation, with particular attention to the effect of cell resting.
Methodology: CD45RA-depleted fractions were obtained from 199 healthy donors. Three sample groups were analyzed: fresh products (n=122), cryopreserved for 2-4 weeks (n=30), and cryopreserved for 5 years (n=15). Cells were rested in CTL Test Medium for 18–24 hours at 37°C before ELISpot analysis. VSTs specific to CMV, EBV, and ADV were quantified using ELISpot assay. The Mon/CD3 ratio was determined by flow cytometry.
Results: Poor detection of VSTs was observed in CD45RA-depleted samples with a Mon/CD3 ratio of 0.38. After cell resting (Mon/CD3=0.05), detection rates increased significantly (CMV = 101, EBV = 78, ADV = 26; p < 0.0001). Following 5-year cryopreservation, despite cell resting, the median frequency of detectable VSTs remained reduced (CMV = 28, EBV = 5, ADV=2 per 300,000 cells).
Conclusion: Cell resting for 18–24 hours is essential for accurate VST quantification in CD45RA-depleted products. ELISpot testing is recommended before infusion of long-stored aliquots to ensure antiviral efficacy.
Introduction
Viral infections caused by cytomegalovirus (CMV), Epstein-Barr virus (EBV), and adenovirus (ADV) are among the leading infectious complications following hematopoietic stem cell transplantation (HSCT)1,2. While antiviral drugs are widely used, their efficacy is often limited by drug resistance, toxicity, and delayed immune reconstitution.
Adoptive transfer of virus-specific T-lymphocytes (VSTs) has emerged as an effective therapeutic approach, capable of reconstituting immunity without exacerbating graft-versus-host disease (GVHD)3. The use of CD45RA-depleted T-cell products allows for the selective administration of memory T cells, reducing the risk of alloreactivity. The effectiveness of this approach has been demonstrated in numerous studies4,5. At the Dmitry Rogachev National Medical Research Center of Pediatric Hematology, Oncology and Immunology, this type of therapy has been successfully applied for more than 10 years6,7.However, a critical factor for the success of this strategy lies in the accurate evaluation of the frequency and function of VSTs in donor products8,9.
This study aimed to assess the detectability of CMV-, EBV-, and ADV-specific T-lymphocytes in CD45RA-depleted donor cell products before and after cryopreservation, and to evaluate the impact of cell resting and long-term storage on VST detection.
Methodology
Peripheral blood samples were collected from 199 healthy donors. CD45RA-depleted fractions (167 samples) were obtained via immunomagnetic separation (CliniMACS, Miltenyi Biotec). These samples were divided into three groups: fresh CD45RA-depleted products (n=122), cryopreserved for 2–4 weeks (n=30), and cryopreserved for 5 years (n=15).
All donors of cell material in our Center sign such informed consent, which fully applies to the samples included in this study. Cryopreservation was performed using a programmable freezer (IceCube, Sy-lab©) at a cooling rate of 1°C/min with phase transition compensation. For cryopreservation, a 7.5% DMSO solution was used. Thawing was carried out in a 37°C water bath. Cell viability was assessed by flow cytometry (Beckman Coulter) using 7-AAD. Cell resting was performed in 6-well Corning plates (2×106 cells/ml, 3 ml per well) in CTL Test Medium for 18–24 hours at 37°C.
VSTs were quantified using IFN-γ ELISPOT assay following in vitro stimulation with CMV pp65, EBV consensus, and AdV5 hexon peptide pools (all reagents Miltenyi Biotec). All stimulators were used at a final concentration of 0.15 nmol/ml, as recommended by the manufacturer. The ELISPOT assay was performed according to the CTL ImmunoSpot reagent manufacturer’s instructions. Briefly, 300,000 cells per well in a 96-well plate (200 µl volume) were incubated for 20–24 hours with stimulating peptides on Human IFN-γ Single-Color ELISPOT plates in CTL Test Medium.
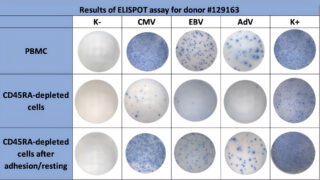
Figure 1. Example of VST detection. Comparison of VST detectability in fresh samples from the same donor: PBMCs, CD45RA-depleted cells without resting, and CD45RA-depleted cells after 18–24 h resting. K− indicates negative control wells, and K+ indicates positive control wells.
After incubation and spot development, the results were analyzed using a CTL S6 Flex M2 ELISPOT Reader. The final values were calculated after subtracting background activity from the negative control (spontaneous activation). Wells stimulated with the polyclonal T-lymphocyte activator phytohemagglutinin (PHA) served as a positive control.
Test results were considered valid if negative control values were <10 spots and positive control values were >100 spots per 300,000 cells per well. Positive response thresholds were defined as >10 spots for CMV and EBV, and >4 spots for AdV per 300,000 mononuclear cells. The cutoff threshold for each pathogen was calculated as the maximum value observed in the negative control (spontaneous activation) + 3SD. The example of VST detection by ELISPOT assay is shown in Figure 1.
Flow cytometry analysis was performed using CD3-APC (clone REA613/SK7), CD14-FITC (clone REA599), and 7-AAD (all reagents from Miltenyi Biotec). The Monocyte (CD14+ cells) to T-lymphocytes (CD3+ cells) ratio (Mon/CD3) was assessed before and after cell resting (BD FACS Canto II).
Statistical analysis was performed in RStudio Server 2024.09.1. Changes in quantities between different time points were assessed using the Mann-Whitney U test for paired data. The level of statistical significance was set at 0.05.
Results
In peripheral blood samples from healthy donors (N=199), pathogen-specific T lymphocytes were readily detectable, with median values of 209 for CMV, 63 for EBV, and 34 for AdV. Cell viability across all groups remained consistently high, ranging between 80–95%. Thus, a sufficient pool of virus-specific cells could be expected in CD45RA-depleted products. Nevertheless, their detectability was sharply reduced after depletion, with median values of only 3, 1, and 0 for CMV, EBV, and AdV, respectively. The median Mon/CD3 ratio in peripheral blood MNCs was 0.12, whereas in fresh CD45RA-depleted fractions it increased to 0.38.
After cell resting, Mon/CD3 ratio was reduced to 0.09 and Median detection rates of VSTs increased significantly: up to 101 for CMV-, 78 for EBV- and 26 for ADV-specific T-lymphocytes per 300,000 MNC (p <0.0001), Figure 2.
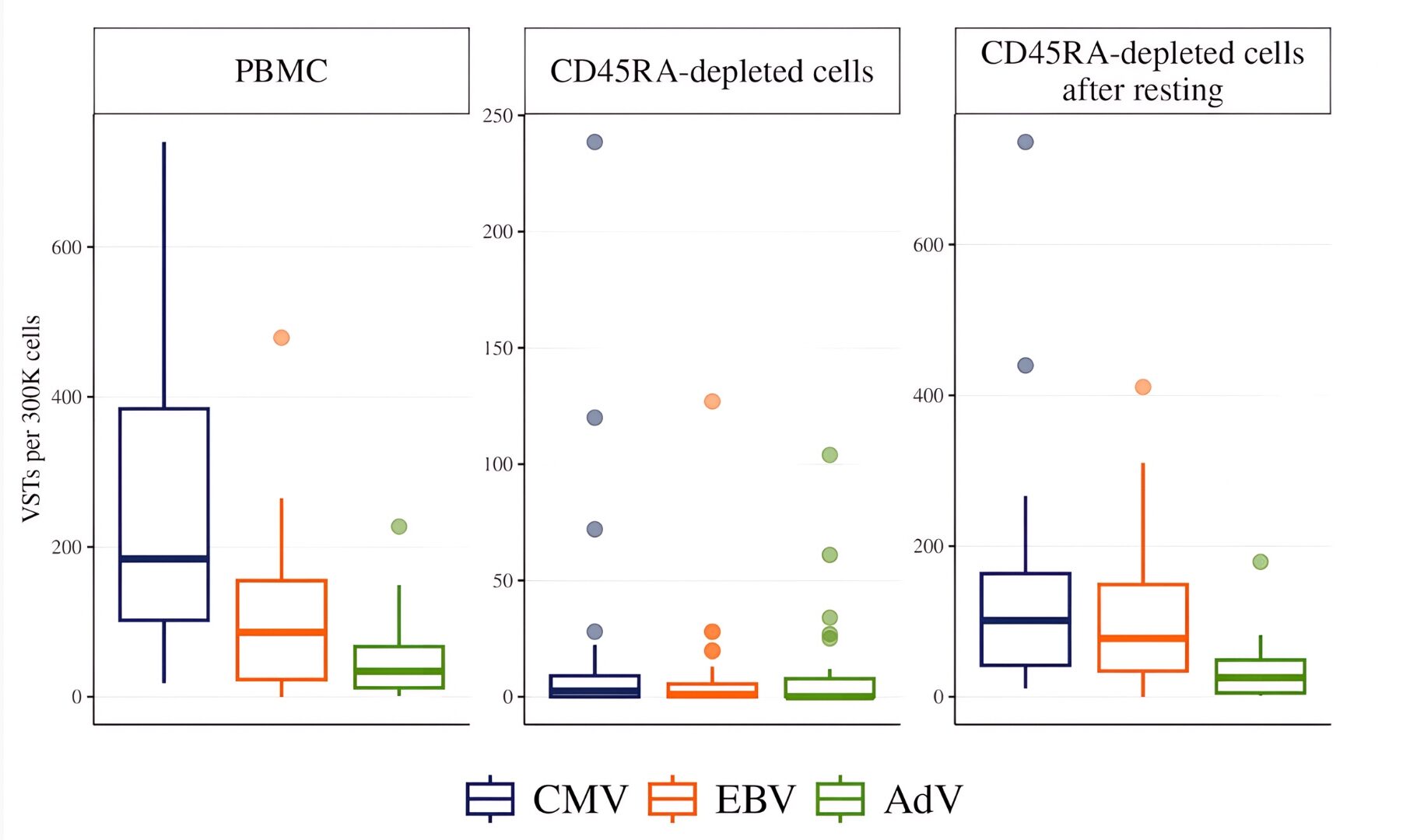
Figure 2. Detection of VST at different stages of processing. The number of IFN-γ–producing cells per 300,000 MNCs was measured by ELISpot in (1) freshly isolated peripheral blood MNC (N=199), (2) CD45RA-depleted fractions (N=122), (3) CD45RA-depleted fractions after 18-24 hours of cell resting (N=122).
In CD45RA-depleted fractions cryopreserved for 2–4 weeks, VST detection was markedly reduced after thawing, with median values of 9, 3, and 1.5 for CMV, EBV, and AdV, respectively. Incorporation of an overnight cell resting step restored VST detectability, raising the medians to 98, 41, and 15, respectively (Figure 3).
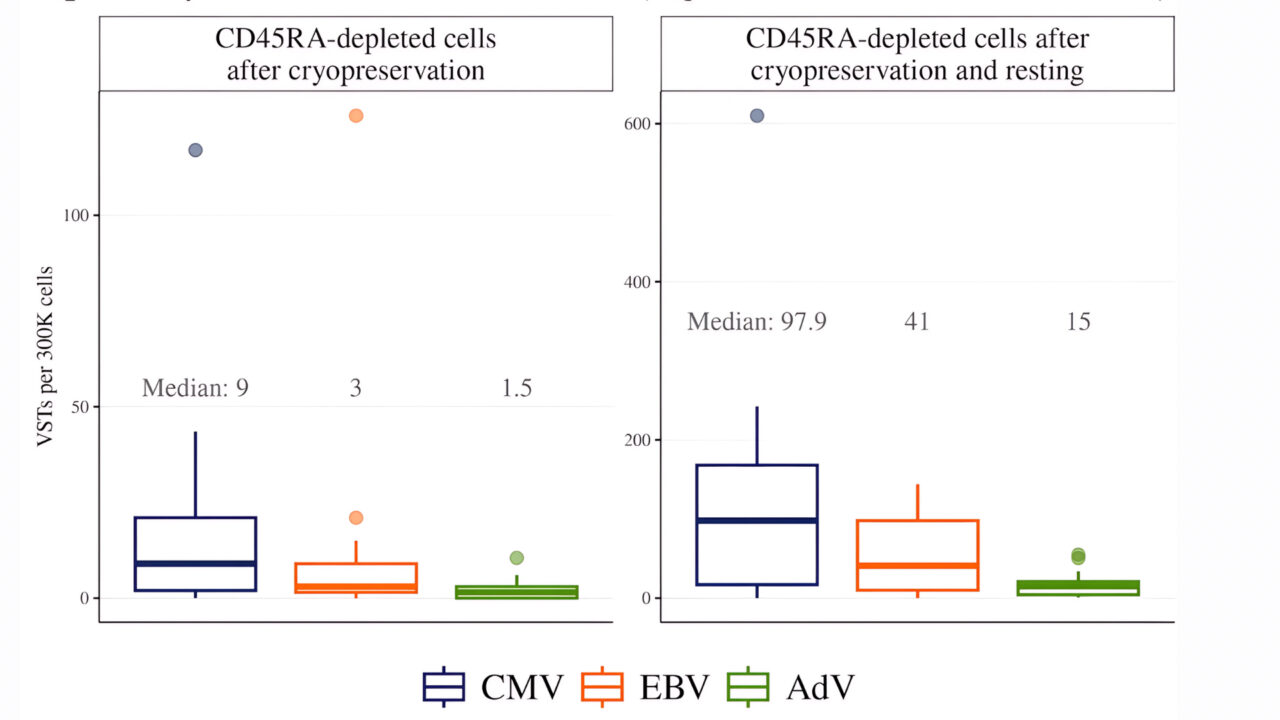
Figure 3. Detection of virus-specific T cells after thawing and after subsequent cell resting. Shown are ELISpot results for CMV-, EBV-, and ADV-specific T cells per 300,000 mononuclear cells. Left panel: detection immediately after thawing (N=30). Right panel: detection after thawing with 18-24 hours of cell resting (N=30).
Table 1 presents the Mon/CD3 ratio in CD45RA-depleted cell fractions at different stages of the experiment.
Table 1. Mon/CD3 ratio in CD45RA-depleted cell fractions at different stages of the experiment
| Condition | N | Median (Q1–Q3) | Range (min–max) | p-value1 | p-value (adjusted)2 |
|---|---|---|---|---|---|
| Fresh, before cryopreservation | 27 | 0.38 (0.22–0.99) | 0.07–3.92 | <0.001 | <0.001 |
| Fresh, after 18–24h of resting | 27 | 0.09 (0.05–0.15) | 0.01–0.31 | <0.001 | <0.001 |
| After thawing (2-4 weeks), no resting | 17 | 0.04 (0.02–0.06) | 0.01–0.61 | 0.03 | 0.03 |
| After thawing (2-4 weeks), after resting | 17 | 0.03 (0.01–0.04) | 0.01–0.17 | 0.03 | 0.03 |
| Fresh, after 18-24h of resting | 15 | 0.02 (0.01–0.04) | 0.01–0.04 | N/A | N/A |
| After thawing + 18-24h resting (>5 years) | 15 | 0.02 (0.01–0.04) | 0.01–0.04 | N/A | N/A |
Table shows Mon/CD3 ratios across different experimental conditions in CD45RA depleted products. Data were obtained by flow cytometry. Values are presented as medians with interquartile ranges (Q1–Q3), as well as minimum and maximum values. Statistical significance (p-value, adjusted p-value) was calculated for paired comparisons as indicated.
1. Mann–Whitney test for paired data
2. p-value adjusted for multiple comparisons using the Holm method
Further detection of VST in CD45RA-depleted products was carried out only after 18-24 hours of resting. Long-term storage (over 5 years) of cryopreserved CD45RA-depleted products significantly reduced VST activity compared to fresh samples and samples after short-term (2–4 weeks) storage. Long-term cryostorage (over 5 years) further diminished VST activity compared to both fresh samples and those stored for 2–4 weeks. Median values decreased from 101 to 28 for CMV, from 78 to 5 for EBV, and from 26 to 2 for AdV (Figures 4–6).
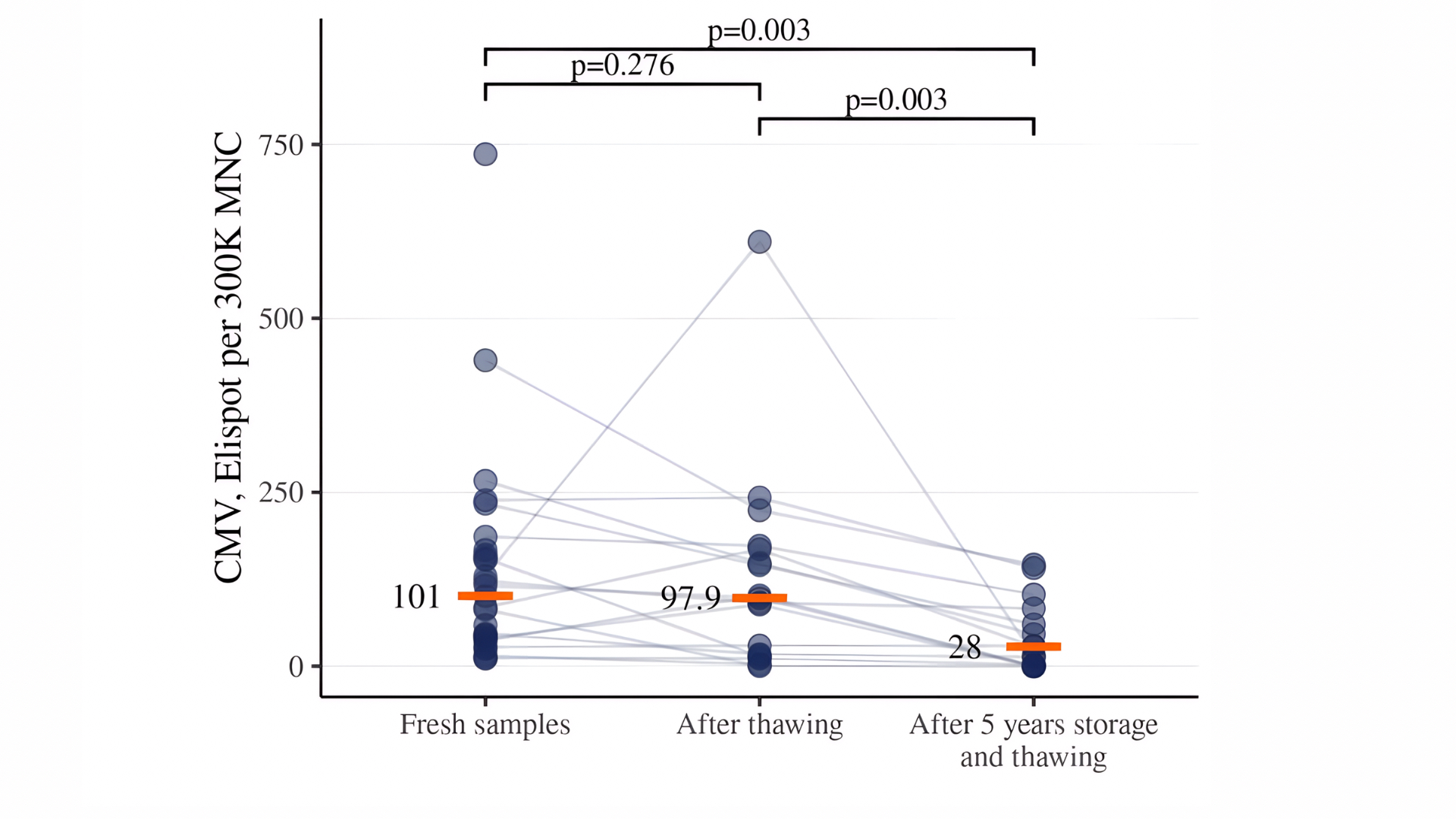
Figure 4. Detection of CMV-specific T cells in CD45RA-depleted products after different storage conditions. Data are presented for fresh samples, cryopreserved samples stored for 2-4 weeks, and for samples stored for 5 years.
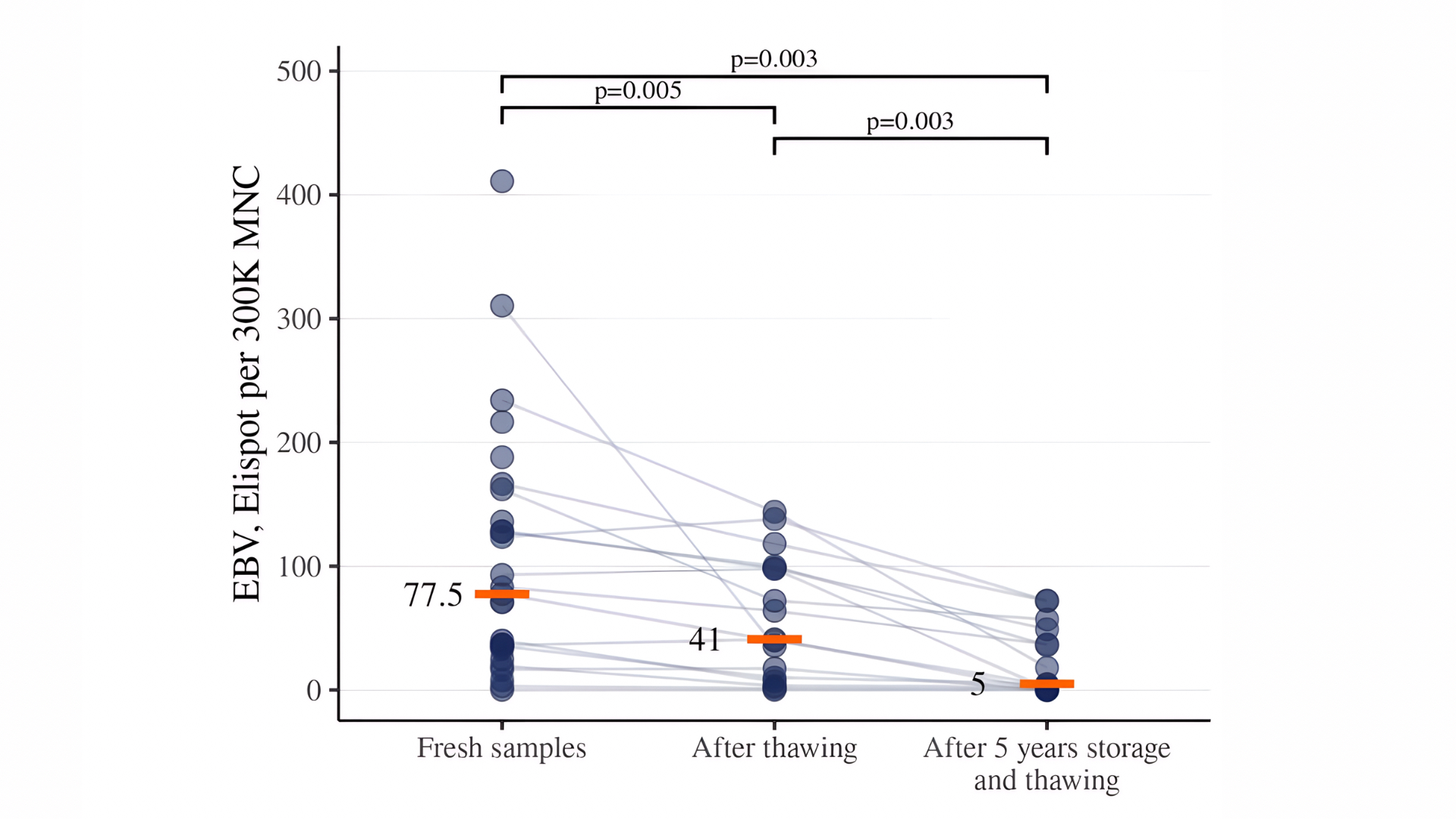
Figure 5. Detection of EBV-specific T cells in CD45RA-depleted products after different storage conditions. Data are presented for fresh samples, cryopreserved samples stored for 2-4 weeks, and for samples stored for 5 years.
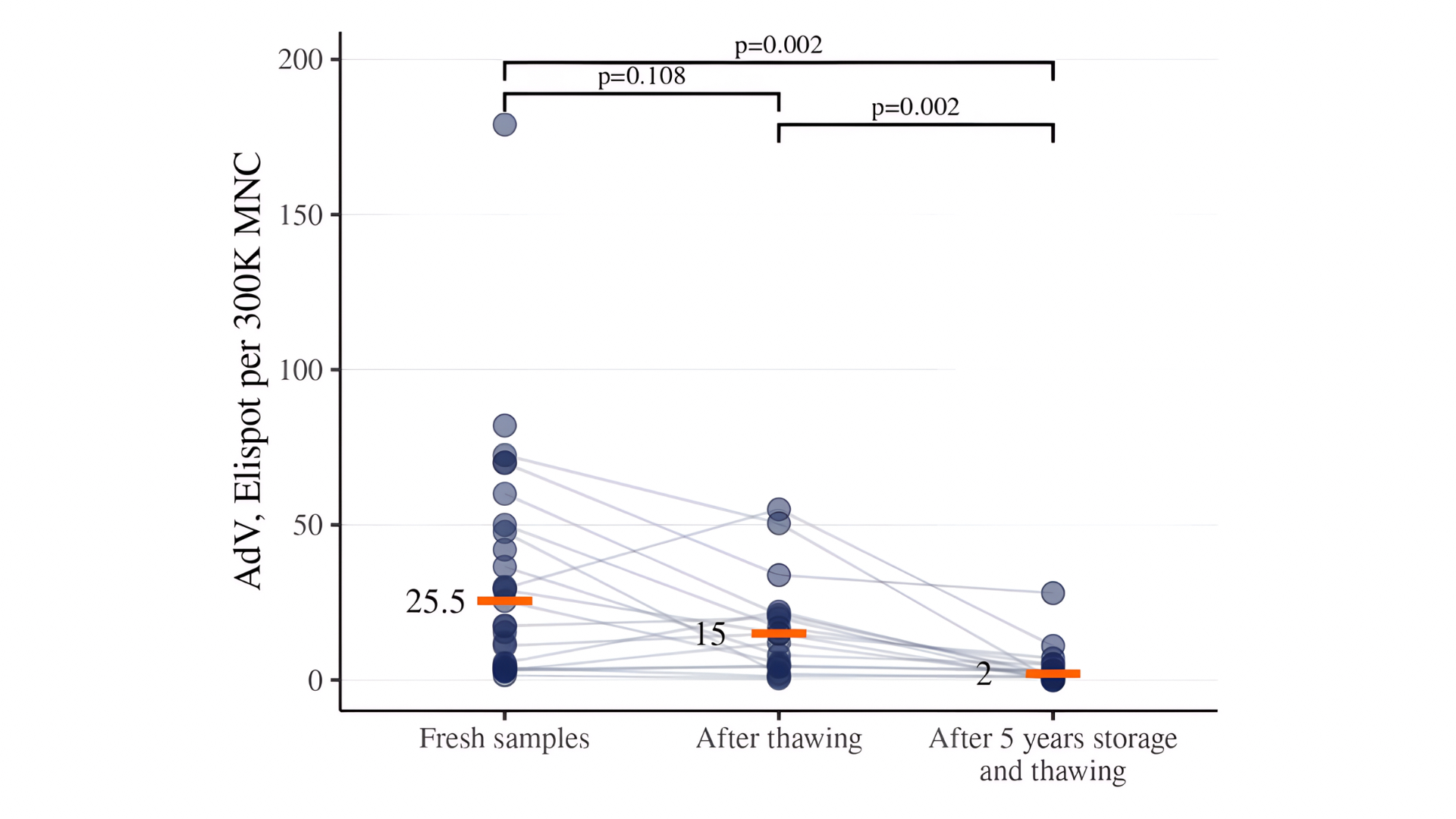
Figure 6. Detection of AdV-specific T cells in CD45RA-depleted products after different storage conditions. Data are presented for fresh samples, cryopreserved samples stored for 2-4 weeks, and for samples stored for 5 years.
Discussion
It is well established that cryopreservation has a significant impact on the functional activity of blood cells10,11. Our findings indicate that monocyte content influences ELISpot-based detection of VSTs in CD45RA-depleted products. However, beyond quantitative effects, functional alterations in monocytes may contribute to suppression of T-cell activation, as suggested by prior reports12. Cell resting appears to restore both the cellular composition and the immunological state of monocytes, suggesting a bidirectional recovery process. Adding cell resting step to the ELISpot assay workflow is necessary for adequate determination of the content of pathogen-specific T-lymphocytes in CD45RA-depleted fractions for cell therapy13.
Despite functional decline due to cryopreservation, VSTs remain detectable even after 5 years of storage. These residual virus-specific cells could be utilized as ‘third-party’ donor material for further expansion, supporting the feasibility of VST manufacturing using long-term cryopreserved products14.
CD45RA-depleted products have long been applied in clinical practice and remain in demand among clinicians. In numerous cases, infusion of CD45RA-depleted donor lymphocyte infusions (DLI) is required at late time points after HSCT. In such scenarios, it is critical to evaluate both the quantity and functional state of memory T cells in the depleted product prior to clinical use15,16. The present study outlines effective laboratory approaches for the detection of pathogen-specific T cells in samples intended for cellular therapy.
Conclusion
Cell resting for 18–24 hours is essential for accurate quantification of virus-specific T-lymphocytes in CD45RA-depleted donor products. Long-term cryopreservation significantly reduces VST functionality, although partial restoration is possible. ELISpot analysis is recommended prior to clinical use of thawed products.
Competing Interests: The authors declare no competing financial interests.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
License
© The Author(s) 2025.
This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, and unrestricted adaptation and reuse, including for commercial purposes, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
To view a copy of this license, visit https://creativecommons.org/licenses/by/4.0/.
References
-
Leen AM, Bollard CM, Mendizabal AM, et al. Multicenter study of banked third-party virus-specific T cells to treat severe viral infections after hematopoietic stem cell transplantation. Blood. 2013 Jun 27;121(26):5113–23.
-
Castagna L, Valli V, Timofeeva I, et al. Feasibility and efficacy of CD45RA+ depleted donor lymphocytes infusion after haploidentical transplantation with post-transplantation cyclophosphamide in patients with hematological malignancies. Transplant Cell Ther. 2021 Jun;27(6):478.e1–5.
-
Hont AB, Powell AB, Sohai DK, et al. The generation and application of antigen-specific T cell therapies for cancer and viral-associated disease. Mol Ther. 2022 Jun 1;30(6):2130–52.
-
Shook DR, Triplett BM, Eldridge PW, et al. Haploidentical stem cell transplantation augmented by CD45RA negative lymphocytes provides rapid engraftment and excellent tolerability. Pediatr Blood Cancer. 2015 Apr;62(4):666-73.
-
Gasior Kabat M, Bueno D, Sisinni L, et al. Selective T-cell depletion targeting CD45RA as a novel approach for HLA-mismatched hematopoietic stem cell transplantation in pediatric nonmalignant hematological diseases. Int J Hematol. 2021 Jul;114(1):116-23
-
Maschan M, Blagov S, Shelikhova L, et al. Low-dose donor memory T-cell infusion after TCR alpha/beta depleted unrelated and haploidentical transplantation: results of a pilot trial. Bone Marrow Transplant. 2018 Mar;53(3):264-73.
-
Dunaikina M, Zhekhovtsova Z, Shelikhova L, et al. Safety and efficacy of the low-dose memory (CD45RA-depleted) donor lymphocyte infusion in recipients of αβ T cell-depleted haploidentical grafts: results of a prospective randomized trial in high-risk childhood leukemia. Bone Marrow Transplant. 2021 Jul;56(7):1614-24.
-
Triplett BM, Muller B, Kang G, et al. Selective T-cell depletion targeting CD45RA reduces viremia and enhances early T-cell recovery compared with CD3-targeted T-cell depletion. Transpl Infect Dis. 2018 Feb;20(1):e12823.
-
Bonifacius A, Lamottke B, Tischer-Zimmermann S, et al. Patient-tailored adoptive immunotherapy with EBV-specific T cells from related and unrelated donors. J Clin Invest. 2023 Jun 15;133(12):e163548.
-
Zhang J, Yin Z, Liang Z, et al. Impacts of cryopreservation on phenotype and functionality of mononuclear cells in peripheral blood and ascites. J Transl Int Med. 2024 Mar 21;12(1):51-63.
-
Luo Y, Wang P, Liu H, et al. The state of T cells before cryopreservation: effects on post-thaw proliferation and function. Cryobiology. 2017 Dec;79:65-70.
-
Sunami K, Teshima T, Nawa Y, et al. Administration of granulocyte colony-stimulating factor induces hyporesponsiveness to lipopolysaccharide and impairs antigen-presenting function of peripheral blood monocytes. Exp Hematol. 2001 Sep;29(9):1117-24.
-
Kuerten S, Batoulis H, Recks MS, et al. Resting of cryopreserved PBMC does not generally benefit the performance of antigen-specific T cell ELISPOT assays. Cells. 2012 Jul 30;1(3):409-27.
-
Devadas SK, Khairnar M, Hiregoudar SS, et al. Is long term storage of cryopreserved stem cells for hematopoietic stem cell transplantation a worthwhile exercise in developing countries? Blood Res. 2017 Dec;52(4):307-10.
-
Triplett BM, Shook DR, Eldridge P, et al. Rapid memory T-cell reconstitution recapitulating CD45RA-depleted haploidentical transplant graft content in patients with hematologic malignancies. Bone Marrow Transplant. 2015 Jul;50(7):968-77.
-
Park HJ, Hong KT, Yun SO, et al. Successful treatment of refractory CMV colitis after haploidentical HSCT with post-transplant cyclophosphamide using CD45RA+ depleted donor lymphocyte infusion. Bone Marrow Transplant. 2020 Aug;55(8):1674-6.





