Muscle-Invasive Bladder Cancer: Evolving Therapies and Real-World Access Gaps in Turkey
Introduction
Bladder cancer, primarily Urothelial Carcinoma (UC), ranks as the ninth most diagnosed cancer worldwide, with approximately 614,000 new cases and 220,000 deaths reported in 2022. It predominantly affects men, where it is the sixth most common cancer globally and the fourth most common cancer in men in Turkey1. Among its three forms—non-muscle invasive, muscle-invasive, and metastatic—each presents distinct tumor biology, clinical characteristics, treatment strategies, and prognoses. Recent advancements in systemic therapies, including immune checkpoint inhibitors, antibody-drug conjugates, and targeted agents, have transformed the management paradigm of Muscle-Invasive Bladder Cancer (MIBC). However, disparities in access to these innovations persist, particularly in low- and middle-income countries (LMICs), including Turkey. This review aims to synthesize recent therapeutic developments in MIBC and evaluate their real-world uptake and access barriers in Turkey.
Methodology
This manuscript represents a narrative landscape review. A non-systematic search of PubMed and Google Scholar was conducted to identify English-language publications between 2015 and 2025. Relevant clinical trials, guidelines, and policy documents focusing on perioperative and metastatic muscle-invasive bladder cancer management were included. National drug approval and reimbursement data of Turkey were also incorporated to contextualize access barriers.
Diagnostic Approach and Staging
Cystoscopy remains the gold standard for diagnosing bladder cancer. When a lesion is detected during cystoscopy, it is typically followed by an examination under anesthesia combined with transurethral resection of bladder tumor (TURBT). Pathological evaluation of patients involves urine-based tests to identify malignant cells and/or histological analysis of biopsy or TURBT specimens from visibly identifiable lesions2.
During the staging evaluation of bladder cancer, cross-sectional abdominopelvic imaging is essential for identifying bladder masses, ideally before TURBT. These imaging studies assist in determining tumor location and disease extent, including possible upper tract involvement, extravesical spread, hydronephrosis, lymphadenopathy, and distant metastases2.
Pelvic MRI with and without contrast can be utilized for enhanced local staging, particularly for assessing the depth of bladder wall invasion. The strongest evidence for MRI use is in the pre-TURBT evaluation of muscle-invasive bladder cancer (MIBC), where multiparametric MRI offers superior soft tissue delineation compared to CT. MRI also holds potential for evaluating treatment response following TURBT, neoadjuvant chemotherapy, or chemoradiation2.
For patients diagnosed with MIBC, a chest CT scan is recommended to assess for pulmonary metastases. Bone scans and brain MRI are generally reserved for symptomatic individuals or those at high risk due to tumor stage, size, or adverse histological features. Although 18F-FDG PET-CT is not routinely employed for localized disease, it may have a role in evaluating locally advanced or suspected metastatic disease2.
Management
Optimal management of bladder cancer requires a multidisciplinary approach. TURBT is a critical procedure for diagnosis, staging, and treatment. In non-muscle-invasive bladder cancer (NMIBC), TURBT aims for complete resection, while in MIBC, it facilitates accurate staging and timely definitive treatment2. Due to its technical complexity and bladder perforation risk, TURBT may not always achieve complete resection of NMIBC. A second TURBT, performed 2–6 weeks later, is recommended if the initial resection is incomplete, in T1 disease, or when detrusor muscle is absent in the specimen, except for Ta low-grade tumors or primary carcinoma in situ (CIS)3.
Muscle Invasive Bladder Cancer (MIBC)
Radical cystectomy, including pelvic lymph node dissection (LND) and urinary diversion, is the standard for localized MIBC and BCG-unresponsive cases4. Two randomized trials investigated the role of extended LND (including the common iliac, presacral and up to, at least, the aortic bifurcation) and found that extended LND was associated with more grade ≥3 complications but no benefit in survival5,6. Selected pT3/T4 pN0–2 cases may benefit from adjuvant pelvic radiotherapy7,8. Addition of adjuvant radiotherapy to chemotherapy alone was associated with improved local relapse-free survival9. Partial cystectomy is an option for carefully selected patients with a single tumor in a favorable location (e.g., bladder dome), requiring meticulous surgical technique to avoid tumor and urine spillage10.
Trimodality therapy (TMT) combines maximal TURBT with chemoradiotherapy. It is a bladder-preserving option, especially for patients who are medically unfit for radical cystectomy2.The BC2001 trial confirmed chemoradiotherapy’s superiority over radiotherapy alone11. Cisplatin or gemcitabine-based regimens are commonly used. Ongoing trials are integrating immunotherapy into TMT12,13. About 10–15% of TMT patients require salvage cystectomy, which carries higher risks. Bladder surveillance is crucial for recurrence detection. When paired with local or metastasis-directed therapy, radiotherapy may also help patients with metastatic bladder cancer or oligometastatic disease. Multidisciplinary collaboration is essential to ensure shared and informed decision-making2.
Perioperative Systemic Therapy
Patients with MIBC face a high risk of metastatic recurrence despite curative-intent local therapy, prompting the exploration of systemic therapies to improve outcomes. In patients with clinical stage T2–T4aN0M0, the BA06 30894 trial revealed that neoadjuvant cisplatin, methotrexate plus vinblastine improved survival (HR 0.84, 95% CI 0.72–0.99)14. The SWOG 8710 trial also reported an improvement in overall survival (OS) with neoadjuvant methotrexate, vinblastine, doxorubicin plus cisplatin (MVAC) (HR 0.75, 95% CI 0.57–1.00). Neoadjuvant cisplatin-based chemotherapy before cystectomy has been shown to increase the likelihood of achieving a pathological complete response compared to cystectomy alone, establishing it as standard care for MIBC. However, the optimal regimen, whether gemcitabine plus cisplatin or dose-dense MVAC, remains a subject of debate15,16.
There has historically been no standard perioperative systemic therapy to decrease the risk of recurrence after curative-intent surgery in cisplatin-ineligible patients with high-risk pathological features at cystectomy or patients who received prior neoadjuvant therapy with high-risk pathological features at cystectomy (pT3 and/or pN+). To determine the role of adjuvant PD1 (Programmed Death-1) or PDL1 (Programmed Death Ligand-1) blockage in this cohort, three phase III trials were conducted.
Checkmate 274 demonstrated a significant improvement in disease-free survival (DFS). In the intention-to-treat population, the median DFS was 20.8 months (95% CI, 16.5–27.6) with nivolumab, compared to 10.8 months (95% CI, 8.3–13.9) with placebo. At 6 months, 74.9% of patients receiving nivolumab were alive and disease-free, versus 60.3% in the placebo group (HR 0.70; 98.22% CI, 0.55–0.90; P<0.001). Among patients with increased PDL1 expression, the percentage of patients was 74.5% and 55.7%, respectively (HR 0.55; 98.72% CI, 0.35-0.85; P<0.001)17.
The IMvigor010 trial did not meet its primary endpoint of improving DFS. Median DFS was 19.4 months (95% CI, 15.9–24.8) with atezolizumab compared to 16.6 months (95% CI, 11.2–24.8) with observation (HR 0.89; 95% CI, 0.74–1.08; p = 0.24)18. However, an exploratory analysis suggested a disease-free and overall survival benefit with adjuvant atezolizumab versus placebo in patients with detectable baseline ctDNA, paving the way for ctDNA-based studies of adjuvant therapy in bladder cancer. Patients who were positive for ctDNA had improved DFS and OS in the atezolizumab arm versus the observation arm (DFS HR 0.58 ,95% CI 0.43-0.79; P = 0.0024, OS HR:0.59 ,95% CI: 0.41-0.86)19.
Recently, the AMBASSADOR trial found that adjuvant pembrolizumab significantly extended disease-free survival compared to observation in patients with high-risk muscle-invasive urothelial carcinoma who were cisplatin-ineligible or declined adjuvant cisplatin-based therapy following radical surgery. The median disease-free survival was 29.6 months (95% CI, 20.0 to 40.7) with pembrolizumab and 14.2 months (95% CI, 11.0 to 20.2) with observation (HR 0.73; 95% CI, 0.59 to 0.90; two-sided P=0.003)20.
In phase 3 NIAGARA study, perioperative durvalumab plus neoadjuvant gemcitabine–cisplatin led to significant improvements in event-free survival (EFS) (67.8% vs 59.8%) and OS (82.2% vs 75.2%) at 24 months as compared with neoadjuvant chemotherapy alone in cisplatin-eligible patients with muscle-invasive bladder cancer. P<0.001 for EFS, P=0.01 for OS21.
Systemic Therapy for Metastatic Bladder Cancer
Cisplatin-based chemotherapy has long been the standard first-line treatment for metastatic urothelial carcinoma. However, advancements have shifted the standard of care, particularly with the addition of novel therapies. Recent phase 3 EV-302 trial established the combination of an antibody–drug conjugate (enfortumab vedotin) and pembrolizumab as a new standard for untreated locally advanced or metastatic urothelial carcinoma. This regimen demonstrated superior PFS and OS compared to platinum-based chemotherapy. Patients treated with the combination showed a median PFS of 12.5 months versus 6.3 months with chemotherapy. Median OS was also significantly longer at 31.5 months versus 16.1 months for chemotherapy. Importantly, treatment-related adverse events of grade ≥3 were less frequent with the combination than with chemotherapy alone22.
In the early 1990s, cisplatin-based combination chemotherapy became the standard treatment for metastatic bladder cancer after a randomized clinical trial demonstrated that MVAC improved survival compared to cisplatin alone23. Reduced toxicity and possibly improved efficacy were observed with dose-dense MVAC administration and/or granulocyte colony-stimulating factor support24,25. Additionally, the combination of gemcitabine and cisplatin demonstrated similar efficacy with lower toxicity than MVAC 26.
Although cisplatin-based chemotherapy became a standard of care for patients with metastatic urothelial cancer, many patients with bladder cancer are of advanced age and many are ineligible for cisplatin. More than 50% of patients are ineligible for cisplatin because of poor performance status (PS), impaired renal function, or comorbidity that forbids high-volume hydration27. According to the Gupta criteria, eligibility for cisplatin-based chemotherapy is assessed based on specific clinical parameters. Cisplatin ineligibility is defined by the presence of at least one of the following conditions: an Eastern Cooperative Oncology Group (ECOG) performance status ≥ 3, creatinine clearance < 30 mL/min, peripheral neuropathy of grade ≥ 2, New York Heart Association (NYHA) class > 3 congestive heart failure, or the combination of ECOG performance status 2 and creatinine clearance < 30 mL/min28. Patients meeting these criteria are deemed ineligible for cisplatin and may instead be considered for carboplatin-based treatment regimens as a therapeutic alternative27.
In recent years, the FDA has approved several immune checkpoint inhibitors targeting PD-1 or PD-L1 for the treatment of metastatic bladder cancer. These agents may be administered as first-line therapy in patients who are ineligible for platinum-based chemotherapy, as maintenance treatment following a response or stable disease after initial platinum-based regimens, or as second-line therapy in cases of disease progression after prior platinum-based chemotherapy29,30. Moreover, the phase 3 CheckMate 901 trial highlighted the efficacy of nivolumab combined with gemcitabine-cisplatin as a first-line option. This combination significantly improved OS (21.7 months vs. 18.9 months) and progression-free survival (PFS 7.9 months vs 7.6 months) compared to gemcitabine-cisplatin alone. The combination also yielded higher objective response rates and longer durations of complete responses31.
The role of maintenance therapy has also been redefined with avelumab in the phase III JAVELIN-Bladder 100 research. Maintenance avelumab after response to first-line chemotherapy significantly improved OS compared to best supportive care. At one year, OS was 71.3% with avelumab versus 58.4% with supportive care, establishing its role as a critical component in the treatment landscape32,33. Additionally in 2017, FDA approved pembrolizumab for patients with platinum-resistant metastatic urothelial cancer based on a randomized phase III trial Keynote-04534.
Erdafitinib is the only tyrosine kinase inhibitor that is currently approved by the FDA for the treatment of advanced or metastatic urothelial carcinoma, despite the fact that numerous additional drugs are being investigated. To be eligible for erdafitinib treatment, patients must have progressed during or after platinum containing chemotherapy, including within 12 months of neoadjuvant or adjuvant platinum based chemotherapy, and have tumors harboring a susceptible FGFR2/3 alterations (fusions or mutations). In THOR Cohort 1, median OS was significantly improved with erdafitinib compared to chemotherapy (12.1 months vs. 7.8 months; HR 0.64; 95% CI, 0.47–0.88; P = 0.005). Median PFS was also longer with erdafitinib (5.6 months vs. 2.7 months; HR 0.58; 95% CI, 0.44–0.78; P <0.001)35.
Fam-trastuzumab deruxtecan was studied in the open-label phase II DESTINY-PanTumor02 trial. Eligibility for this trial included a HER2 expressing tumor (IHC 3+/2+ by local or central testing) with locally advanced or metastatic disease after at least 1 systemic treatment or without alternative treatments. For the 41 patients with bladder cancer, ORR was 39.0%, median PFS was 7.0 months, and median OS was 12.8 months. Grade ≥3 drug-related AEs were observed in 40.8% of all patients on the study, including 10.5% with adjudicated drug-related interstitial lung disease, of which 3 patients (1.1%) died36.
Current Treatment Landscape in Turkey
Recent years have witnessed a paradigm shift in the management of muscle-invasive and metastatic urothelial carcinoma (mUC), with the approval of immune checkpoint inhibitors (ICIs), antibody-drug conjugates (ADCs), and targeted therapies such as FGFR inhibitors. However, these advances remain largely inaccessible in Turkey like other LMICs due to regulatory delays, lack of reimbursement policies, limited health infrastructure, and socioeconomic barriers.
In Turkey, for MIBC patients, those unable or unwilling to undergo surgery but achieve complete tumor removal through TURBT are treated with chemoradiotherapy. Locally advanced cases typically receive neoadjuvant chemotherapy, predominantly gemcitabine and cisplatin, to optimize surgical outcomes before radical cystectomy, which remains the standard of care for eligible patients (see Figure 1).
Figure 1: Management of patients with histopathologically confirmed bladder cancer
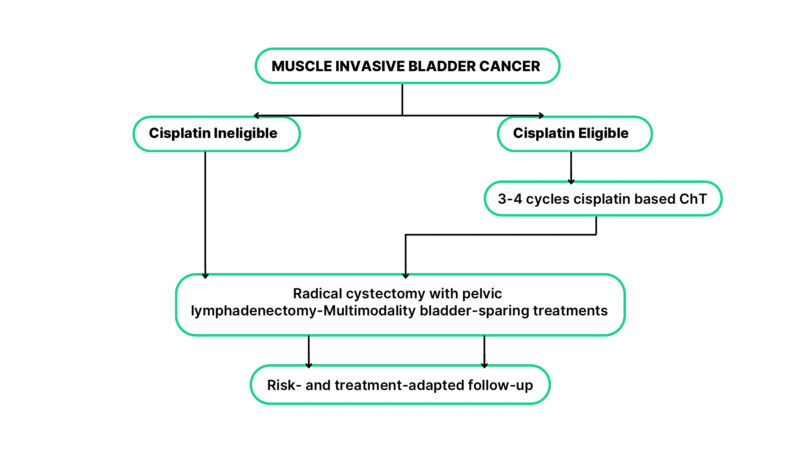
* Adopted from: Bladder Cancer: ESMO Clinical Practice Guideline for diagnosis, treatment, and follow-up (2022)
However, disparities in access to neoadjuvant chemotherapy persist across many regions globally, underscoring inequalities in care. A recent analysis of over 25,000 patients with muscle-invasive bladder cancer (MIBC) from the National Cancer Database (NCDB) demonstrated that lower-income patients were significantly less likely to receive neoadjuvant chemotherapy (NAC) and adequate pelvic lymph node dissection (PLND) during radical cystectomy. Importantly, these disparities translated into clinically meaningful differences in survival outcomes; the median OS was 55.9 months in the lowest income quartile compared to 68.2 months in the highest (p <0.001). Even after adjusting for clinical and demographic variables, income independently predicted both treatment receipt and survival outcomes37.
Maintenance avelumab is a critical immunotherapy option in many countries, including Turkey. The only immunotherapy that is reimbursed in Turkey for the treatment of bladder cancer is Avelumab. Nonetheless, the high cost of newer therapies, such as perioperative durvalumab combined with chemotherapy or adjuvant nivolumab for cisplatin-ineligible patients, prevents widespread adoption as these treatments are often not state-funded (see Table 1).
Table 1: Estimated Annual Cost of Non-Reimbursed Therapies for Bladder Cancer Treatment in Turkey
| Drug / Regimen | Cost Per Cycle (₺) | Annual Cost (₺) | Annual Cost (USD) |
|---|---|---|---|
| Durvalumab 1500 Mg (Up To 12 Cycles, Perioperative) | 223.200 | 2.678.400 | 66.000 |
| Nivolumab 240 Mg | 91.300 | 2.374.500 | 58.500 |
| *Enfortumab Vedotin (1.25 Mg/Kg) + Pembrolizumab 200 Mg | 310.200 | 5.273.800 | 130.000 |
| Pembrolizumab 200 Mg | 157.000 | 2.669.700 | 65.800 |
| Trastuzumab Deruxtecan 400 Mg | 134.100 | 2.281.200 | 56.200 |
| *Erdafitinib 8 Mg/Day (Per Month) | 581.000 | 6.971.600 | 171.800 |
1 USD: 40,56 ₺ (30.07.2025)
GDP per capita (~$15,461)
Monthly minimum wage (~$550 USD)
₺: Turkish lira, USD: United States dollar, mg: milligram, kg: kilogram
*
*The dosage was calculated based on a 70-kg individual.
** 12 cycles dosage
Financial barriers also limit the use of breakthrough therapies, including enfortumab vedotin combined with pembrolizumab or pembrolizumab alone. While these treatments have demonstrated significant survival benefits, their high cost restricts accessibility, leaving many patients worldwide without potentially life-saving options. Furthermore, innovative agents such as erdafitinib and trastuzumab-deruxtecan , which target specific molecular subtypes of bladder cancer, remain non-reimbursed in Turkey (see Table 1).
In the second-line and subsequent treatment settings, therapeutic options in Turkey are limited to conventional cytotoxic agents such as docetaxel, paclitaxel, gemcitabine, and vinflunine, highlighting the absence of access to newer, more effective agents in later lines of therapy. To the best of our knowledge, no prior studies have specifically addressed access to novel therapies for bladder cancer in Turkey; therefore, current utilization rates and treatment proportions remain unknown. Consequently, while detailed data are lacking, a treatment algorithm has been summarized based on agents that are currently approved and reimbursed in Turkey (see Figure 2).
Figure 2: Treatment algorithm based on drugs that are approved and reimbursed in Turkey
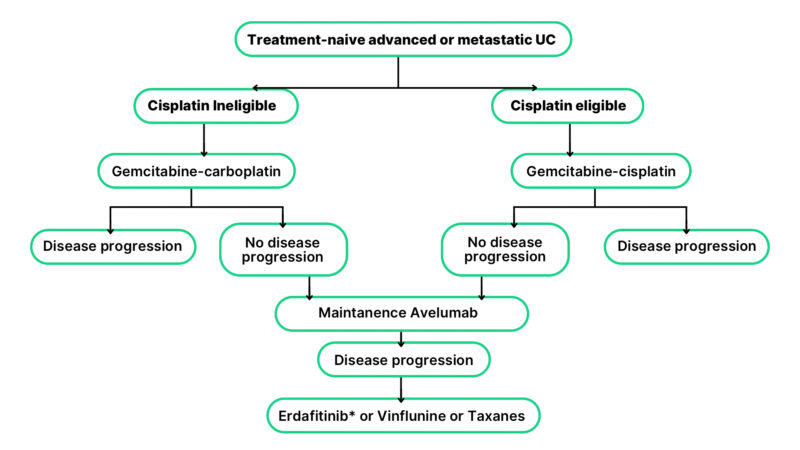
* Erdafitinib is approved in Turkey but not reimbursed
The differences in regulatory approvals and reimbursement status of novel therapeutic agents between Turkey, the European Medicines Agency (EMA), and the U.S. Food and Drug Administration (FDA) are summarized in Table 2. Turkey’s slower integration of novel therapies stems from delayed regulatory approvals, budget constraints, and limited biomarker infrastructure. Aligning local reimbursement strategies with EMA guidance could help bridge this gap.
Table 2: Regulatory Approval and Reimbursement Comparison of Selected New Therapies for MIBC (as of July 2025)
| Treatment Plan | Drug / Combination | Indication | FDA Approval | EMA Approval | TMMDA Approval, Turkey | Reimbursement, Turkey |
|---|---|---|---|---|---|---|
| Perioperative | Cisplatin + Gemcitabin + Durvalumab | Perioperative, MIBC | Yes (2025) | Yes (2025) | No | No |
| Adjuvant | Nivolumab (Single agent) | Adjuvant, mUC | Yes (2021) | Yes (2022) | No | No |
| Metastatic | Nivolumab (Single agent) | mUC after failure of prior platinum-containing therapy | Yes (2017) | Yes (2017) | No | No |
| Metastatic | Avelumab (maintenance) | Post-platinum responsive mUC – maintenance | Yes (2020) | Yes (2021) | Yes (2019) | Yes (2019) |
| Metastatic | Enfortumab Vedotin + Pembrolizumab | 1st line mUC | Yes (2023) | Yes (2024) | No | No |
| Metastatic | Cisplatin + Gemcitabin + Nivolumab | 1st line mUC | Yes (2024) | Yes (2024) | No | No |
| Metastatic | Pembrolizumab (single agent) | Cisplatin ineligible 1stline mUC or second line | Yes (2017) | Yes (2017) | Yes (2021) | No |
| Metastatic | Trastuzumab Deruxtecan | HER2+ solid tumors (including mUC) | Yes (2024) | Yes (2024) | No | No |
| Metastatic | Erdafitinib | FGFR2/3+ mUC (after 2nd line ) | Yes (2019) | Yes (2019) | Yes (2022) | No |
Abbreviations: MIBC: muscle-invasive bladder cancer, mUC: metastatic urothelial carcinoma , FDA: Food and Drug Administration, EMA: European Medicines Agency , TMMDA: Turkish Medicines and Medical Devices Agency
Table 1 provides a summary of the estimated annual costs, in both Turkish lira (₺) and US Dollars (USD), for therapeutic agents that are currently not covered by reimbursement in Turkey. In Turkey, the estimated annual cost of novel therapies exceeds both the country’s GDP per capita (~$15,461) and is more than 100 to 300 times the monthly minimum wage (~$550 USD)38.
Given the challenges in accessing novel therapies in Turkey, as outlined above and in Table 1, efforts have been made to increase patient access to current treatment options by referring eligible individuals to clinical trials. Patients from Turkey have been enrolled in pivotal clinical trials demonstrating clinically meaningful outcomes, including EV-302, CheckMate 901, and NIAGARA. Additionally, an early access program for avelumab was recently conducted to facilitate patient access to the drug, and the findings from this initiative have been published in a recent study39.
Globally, the accessibility of advanced treatments for bladder cancer remains a critical challenge, significantly impacting patient outcomes. A study examining the barriers to new systemic treatments for genitourinary cancers in low- and middle-income countries (LMICs) found that access was severely restricted, particularly for patients dependent on the public healthcare system, by the absence of novel systemic agents such as immunotherapies and targeted therapies. Access to treatment was also found to be negatively impacted by the independent administration of drug approval procedures and reimbursement regulations40.
Conclusion
Improving access to advanced therapies is essential not only for Turkey but also for many countries worldwide. Expanding public coverage of innovative treatments and adopting cost-effective strategies within healthcare systems are key to achieving equitable care. Such global efforts can improve patient outcomes, reduce treatment disparities, and help align national standards with the latest advances in oncology.
Limitations
This review is narrative in nature and does not follow the systematic methodology of a formal systematic review or meta-analysis, which may introduce selection bias in the inclusion of studies and data sources. While an effort was made to include the most relevant and up-to-date literature, some emerging data or unpublished findings may not have been captured. Additionally, treatment access data specific to Turkey are limited due to the lack of nationwide registry or published utilization rates, particularly for recently approved agents. Consequently, real-world treatment patterns and uptake rates could not be quantitatively assessed. These limitations should be considered when interpreting the findings and recommendations presented in this review.
Conflict of Interests: Dr. Yüksel Ürün has served on advisory boards for Abdi-İbrahim, Astellas, AstraZeneca, Bayer, Bristol Myers-Squibb, Eczacıbaşı, Gen ilaç, GSK, Janssen, Merck, MSD, Nobel, Novartis, Pfizer, and Roche. None of these companies have any conflicts of interest related to the subject matter of this review.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
License
© Author(s) 2025.
This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, and unrestricted adaptation and reuse, including for commercial purposes, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
To view a copy of this license, visit https://creativecommons.org/licenses/by/4.0/.
References
-
Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229-63.
-
Dyrskjot L, Hansel DE, Efstathiou JA, Knowles MA, Galsky MD, Teoh J, et al. Bladder cancer. Nat Rev Dis Primers. 2023;9(1):58.
-
Babjuk M, Burger M, Capoun O, Cohen D, Comperat EM, Dominguez Escrig JL, et al. European Association of Urology guidelines on non-muscle-invasive bladder cancer (Ta, T1, and carcinoma in situ). Eur Urol. 2022;81(1):75-94.
-
Witjes JA, Bruins HM, Cathomas R, Comperat EM, Cowan NC, Gakis G, et al. European Association of Urology guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2020 guidelines. Eur Urol. 2021;79(1):82-104.
-
Gschwend JE, Heck MM, Lehmann J, Rubben H, Albers P, Wolff JM, et al. Extended versus limited lymph node dissection in bladder cancer patients undergoing radical cystectomy: survival results from a prospective, randomized trial. Eur Urol. 2019;75(4):604-11.
-
Lerner SP, Tangen C, Svatek RS, Daneshmand S, Pohar KS, Skinner EC, et al. SWOG S1011: A phase III surgical trial to evaluate the benefit of a standard versus an extended lymphadenectomy performed at time of radical cystectomy for muscle invasive urothelial cancer. J Clin Oncol. 2023;41(16).
-
Baumann BC, He J, Hwang WT, Tucker KN, Bekelman JE, Herr HW, et al. Validating a local failure risk stratification for use in prospective studies of adjuvant radiation therapy for bladder cancer. Int J Radiat Oncol Biol Phys. 2016;95(2):703-6.
-
Baumann BC, Bosch WR, Bahl A, Birtle AJ, Breau RH, Challapalli A, et al. Development and validation of consensus contouring guidelines for adjuvant radiation therapy for bladder cancer after radical cystectomy. Int J Radiat Oncol Biol Phys. 2016;96(1):78-86.
-
Zaghloul MS, Christodouleas JP, Smith A, Abdallah A, William H, Khaled HM, et al. Adjuvant sandwich chemotherapy plus radiotherapy vs adjuvant chemotherapy alone for locally advanced bladder cancer after radical cystectomy: a randomized phase 2 trial. JAMA Surg. 2018;153(1):e174591.
-
Peak TC, Hemal A. Partial cystectomy for muscle-invasive bladder cancer: a review of the literature. Transl Androl Urol. 2020;9(6):2938-45.
-
James ND, Hussain SA, Hall E, Jenkins P, Tremlett J, Rawlings C, et al. Radiotherapy with or without chemotherapy in muscle-invasive bladder cancer. N Engl J Med. 2012;366(16):1477-88.
-
Gupta S, Fujii Y, van der Heijden MS, Weickhardt AJ, James ND, Shariat SF, et al. Phase 3 KEYNOTE-992 study of pembrolizumab plus chemoradiotherapy versus placebo plus chemoradiotherapy in patients with muscle-invasive bladder cancer (MIBC). J Clin Oncol. 2024;42(4):TPS720-TPS.
-
Singh P, Tangen C, Efstathiou JA, Lerner SP, Jhavar SG, Hahn NM, et al. INTACT: Phase III randomized trial of concurrent chemoradiotherapy with or without atezolizumab in localized muscle invasive bladder cancer—SWOG/NRG1806. J Clin Oncol. 2020;38(6).
-
International Collaboration of T, MRC Advanced Bladder Cancer Working Party, EORTC GU Group, Australian Bladder Cancer Study Group, NCIC Clinical Trials Group, et al. International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. J Clin Oncol. 2011;29(16):2171-7.
-
Grossman HB, Natale RB, Tangen CM, Speights VO, Vogelzang NJ, Trump DL, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349(9):859-66.
-
Advanced Bladder Cancer Meta-analysis Collaboration. Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data. Eur Urol. 2005;48(2):202-5.
-
Bajorin DF, et al. Adjuvant nivolumab versus placebo in muscle-invasive urothelial carcinoma. N Engl J Med. 2021;385(9):864-74.
-
Bellmunt J, Hussain M, Gschwend JE, Albers P, Oudard S, Castellano D, et al. Adjuvant atezolizumab versus observation in muscle-invasive urothelial carcinoma (IMvigor010): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2021;22(4):525-37.
-
Powles T, Assaf ZJ, Davarpanah N, Banchereau R, Szabados BE, Yuen KC, et al. ctDNA guiding adjuvant immunotherapy in urothelial carcinoma. Nature. 2021;595(7867):432-7.
-
Apolo AB, Ballman KV, Sonpavde G, Berg S, Kim WY, Parikh R, et al. Adjuvant pembrolizumab versus observation in muscle-invasive urothelial carcinoma. N Engl J Med. 2025;392(1):45-55.
-
Powles T, Catto JWF, Galsky MD, Al-Ahmadie H, Meeks JJ, Nishiyama H, et al. Perioperative durvalumab with neoadjuvant chemotherapy in operable bladder cancer. N Engl J Med. 2024;391(19):1773-86.
-
Powles T, Valderrama BP, Gupta S, Bedke J, Kikuchi E, Hoffman-Censits J, et al. Enfortumab vedotin and pembrolizumab in untreated advanced urothelial cancer. N Engl J Med. 2024;390(10):875-88.
-
Loehrer PJ, Einhorn LH, Elson PJ, Crawford ED, Kuebler P, Tannock I, et al. A randomized comparison of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. J Clin Oncol. 1992;10(7):1066-73.
-
Sternberg CN, de Mulder P, Schornagel JH, Theodore C, Fossa SD, van Oosterom AT, et al. Seven-year update of an EORTC phase III trial of high-dose intensity M-VAC chemotherapy and G-CSF versus classic M-VAC in advanced urothelial tract tumours. Eur J Cancer. 2006;42(1):50-4.
-
Gabrilove JL, Jakubowski A, Scher H, Sternberg C, Wong G, Grous J, et al. Effect of granulocyte colony-stimulating factor on neutropenia and associated morbidity due to chemotherapy for transitional-cell carcinoma of the urothelium. N Engl J Med. 1988;318(22):1414-22.
-
von der Maase H, Sengelov L, Roberts JT, Ricci S, Dogliotti L, Oliver T, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin with methotrexate, vinblastine, doxorubicin plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23(21):4602-8.
-
De Santis M, Bellmunt J, Mead G, Kerst JM, Leahy M, Maroto P, et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer unfit for cisplatin-based chemotherapy: EORTC study 30986. J Clin Oncol. 2012;30(2):191-9.
-
Gupta S, Bellmunt J, Plimack ER, Sonpavde GP, Grivas P, Apolo AB, et al. Defining platinum-ineligible patients with metastatic urothelial cancer. J Clin Oncol. 2022;40(16_suppl):4577.
-
National Comprehensive Cancer Network. NCCN guidelines: bladder cancer. Version 1.2025 [Internet]. Plymouth Meeting (PA): NCCN; 2025 [updated 2025 Jul 29; cited 2025 Aug 25].
-
Powles T, Bellmunt J, Comperat E, De Santis M, Huddart R, Loriot Y, et al. ESMO clinical practice guideline interim update on first-line therapy in advanced urothelial carcinoma. Ann Oncol. 2024;35(6):485-90.
-
van der Heijden MS, Sonpavde G, Powles T, Necchi A, Burotto M, Schenker M, et al. Nivolumab plus gemcitabine-cisplatin in advanced urothelial carcinoma. N Engl J Med. 2023;389(19):1778-89.
-
Powles T, Park SH, Voog E, Caserta C, Valderrama BP, Gurney H, et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med. 2020;383(13):1218-30.
-
Powles T, Park SH, Caserta C, Valderrama BP, Gurney H, Ullen A, et al. Avelumab first-line maintenance for advanced urothelial carcinoma: results from the JAVELIN Bladder 100 trial after ≥2 years of follow-up. J Clin Oncol. 2023;41(19):3486-92.
-
Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376(11):1015-26.
-
Loriot Y, Matsubara N, Park SH, Huddart RA, Burgess EF, Houede N, et al. Erdafitinib or chemotherapy in advanced or metastatic urothelial carcinoma. N Engl J Med. 2023;389(21):1961-71.
-
Meric-Bernstam F, Makker V, Oaknin A, Oh DY, Banerjee S, Gonzalez-Martin A, et al. Efficacy and safety of trastuzumab deruxtecan in patients with HER2-expressing solid tumors: primary results from the DESTINY-PanTumor02 phase II trial. J Clin Oncol. 2024;42(1):47-58.
-
Antar RM, Xu VE, Adesanya O, Drouaud A, Longton N, Gordon O, et al. Income disparities in survival and receipt of neoadjuvant chemotherapy and pelvic lymph node dissection for muscle-invasive bladder cancer. Curr Oncol. 2024;31(5):2566-81.
-
The World Bank. The World Bank in Türkiye [Internet]. Washington (DC): The World Bank; c2025 [updated 2025 Apr 24; cited 2025 Aug 25].
-
Tural D, Özkan O, Yaslikaya S, Tatli AM, Akdag G, Demir H, et al. Avelumab maintenance in patients with metastatic urothelial carcinoma in a real-life expanded-access program (EAP). J Clin Oncol. 2025;43(5_suppl).
-
Herchenhorn D, Freire V. Access of new systemic therapies for genito-urinary cancers in low-middle income countries. Front Urol. 2022;2:1-8.





