Gastric Cancer in Latin America
Abstract
Gastric cancer (GC) remains a significant health burden in Latin America, marked by notable regional disparities in incidence, diagnosis, and treatment. This review highlights the multifaceted challenges and variations across different countries within the region. High-incidence countries like Peru and Chile contrast sharply with lower-incidence nations such as Caribbean countries, reflecting differences in Helicobacter pylori prevalence, dietary habits, and genetic factors. Diagnostic challenges are pronounced in some areas, where the availability of endoscopy, trained healthcare professionals, and advanced diagnostic tools is limited, leading to delayed diagnoses and advanced-stage presentations.
Treatment options also reveal significant disparities. For localized and resectable locally advanced tumors, potentially curative gastrectomy with D2 lymphadenectomy is the cornerstone, yet it is offered mainly in high-volume urban centers. For metastatic disease, standard chemotherapy is the primary treatment modality, while access to innovative therapies such as immunotherapy and Claudin inhibitors remains scarce. This lack of advanced treatments limits the prognosis for many patients. The overall 5-year survival rate in Latin America remains low, emphasizing the need for tailored public health interventions to address these disparities.
This review includes an overview of the epidemiology, clinical presentation, treatment, and survival of gastric cancer in Latin America, emphasizing the need for improved diagnostic and therapeutic resources. It aims to highlight the urgent necessity for tailored public health interventions to reduce the burden of gastric cancer and improve patient outcomes across the continent.
Introduction
Gastric cancer represents the fifth most common malignancy and the third leading cause of cancer-related deaths worldwide, with approximately 1 million new cases and 660,175 deaths estimated in 2022 according to the Global Cancer Observatory1,2.
In Latin America (LATAM), this disease poses a significant challenge, accounting for a considerable share of the global burden, due to factors such as the high prevalence of Helicobacter pylori (H. pylori) and disparities in healthcare systems3. The region has significant geographic and socioeconomic variations that influence the risk of developing the disease and opportunities for early detection.
This review shares the differences in the epidemiology, risk factors, diagnostic methods, and real-world treatment data of gastric cancer in LATAM.
Methodology
We conducted a structured, non-systematic search of PubMed/MEDLINE; the ASCO and ESMO abstract libraries; SciELO; and regional/non-indexed sources (e.g., LILACS, national cancer registries, Ministry of Health reports, and institutional repositories). We included all peer-reviewed articles and conference abstracts reporting data on incidence, sociodemographic and clinical-pathological characteristics, as well as information on treatment strategies and oncological outcomes of patients from any LATAM country. As this is a narrative review, we did not perform duplicate independent screening or formal risk-of-bias scoring.
Epidemiology in Latin America
Gastric cancer has a high incidence in LATAM, being the fifth most common malignancy with 74,257 new cases per year and ranking as one of the leading causes of cancer-related deaths in several countries in the region. Additionally, it accounts for approximately 57,895 annual deaths in LATAM, with an incidence-to-mortality ratio exceeding 70%, indicating predominantly late-stage diagnoses1,2.
Incidence and mortality vary strikingly across Latin America, shaped by geography, socioeconomic conditions, and the uneven reach of health services (Figures 1 and 2). Outside the traditional Asian hotspots, Japan, Mongolia, and Korea, Peru and Chile have one of the highest incidence rates worldwide, with age-standardized incidences of 14.3 and 14.2 per 100,000 and mortality close behind at 11.1 per 100,000. Costa Rica, Colombia, Ecuador, Haiti, and Guatemala follow, each logging 10-14 cases per 100,000. Mexico and Argentina report lower figures (≈ 6 to 7 per 100,000), yet their incidence-to-mortality ratios remain unfavorable, a sign that most tumors are still caught late.
The Caribbean presents a contrasting picture. Islands such as Trinidad and Tobago and Puerto Rico record fewer than five cases per 100,000. Part of the explanation lies in biology: Helicobacter pylori exposure is both less common and acquired later (Puerto Rico’s seroprevalence is just 33 %, like mainland USA4. The rest is methodological: only 12 of 30 Caribbean states maintain cancer registries, and just four meet international quality standards, covering barely 14 % of the region’s population5. Mortality rates have a similar pattern to incidence across all LATAM countries2.
In addition to the differences that exist between countries, there are variations in epidemiology within each country. It is reported that the highest rates of gastric cancer are observed in rural areas such as the Andes or rural regions of Mexico and Central America, where the prevalence of H. pylori can be as high as 80%3. The unequal access to healthcare in these areas further contributes to the high mortality rates of gastric cancer.
Figure 1. Incidence of Gastric Cancer in Latin America (Age-Standardized Rate per 100,000, Both sexes, in 2022)
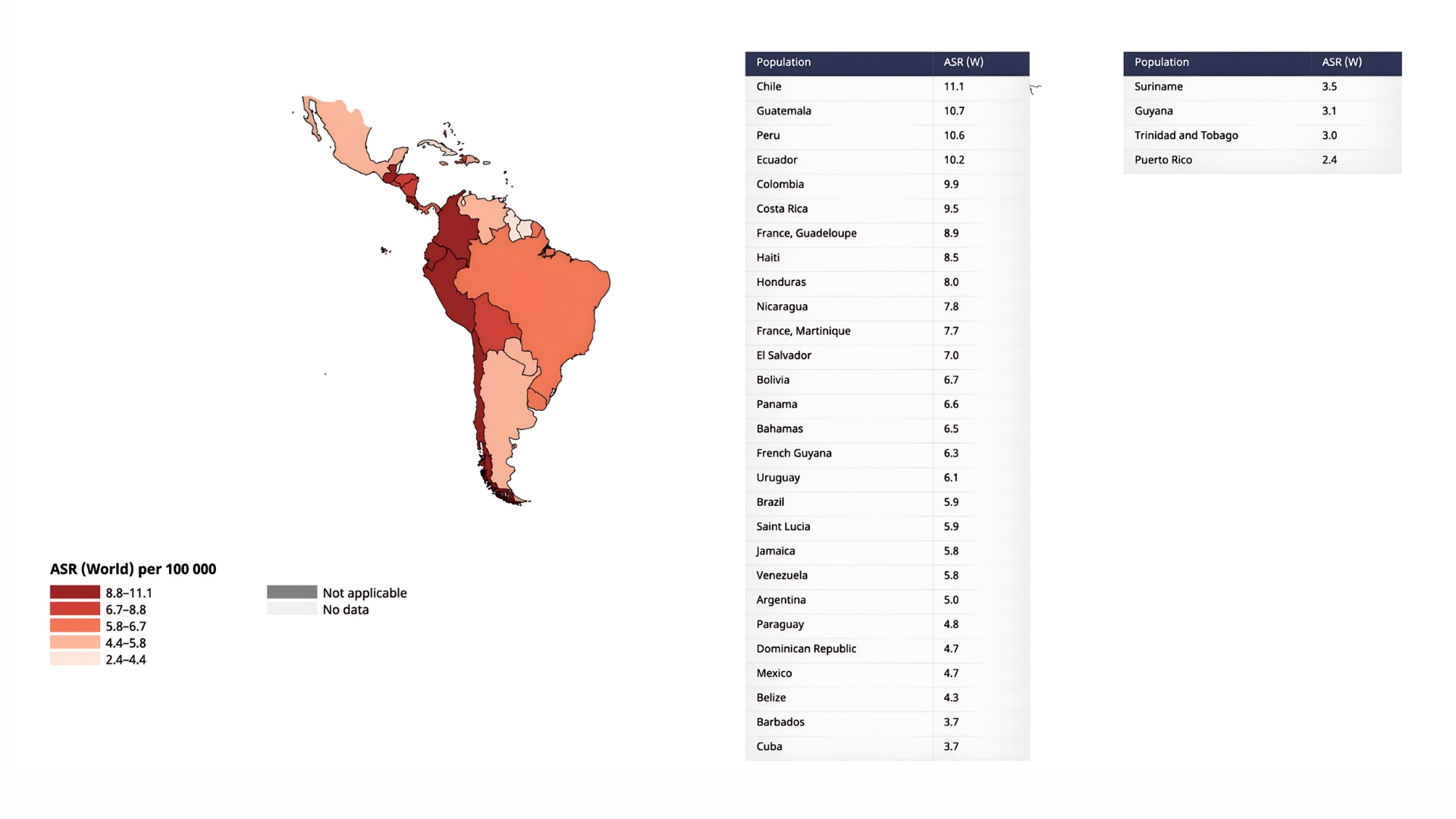
Legend: Taken from Cancer Today / International Agency for Research on Cancer / World Health Organization / Data version: Globocan 2022 (version 1.1) – 08.02.20242.
Figure 2. Mortality of Gastric Cancer in Latin America (Age-Standardized Rate per 100,000, Both sexes, in 2022)
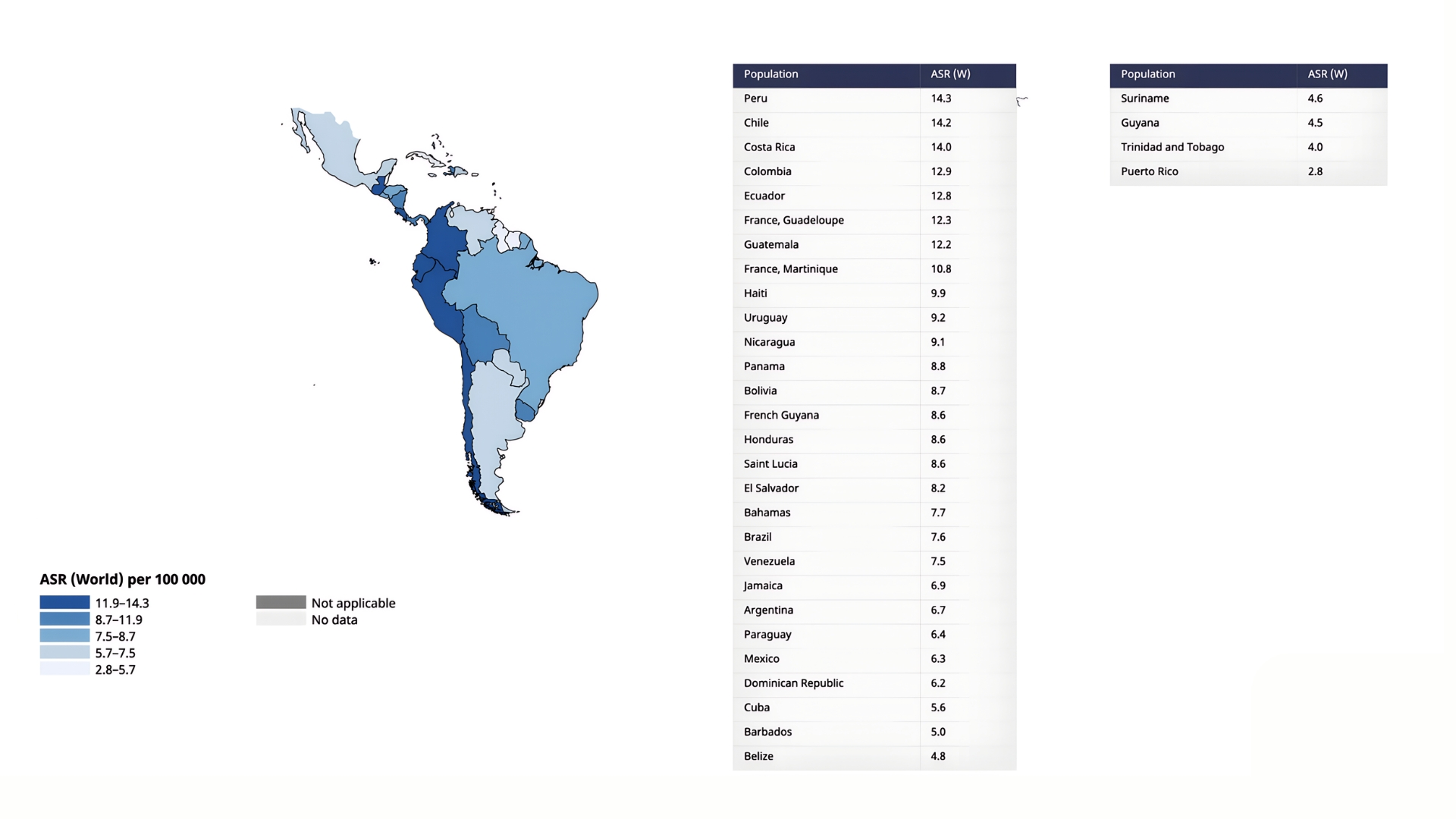
Legend: Taken from Cancer Today / International Agency for Research on Cancer / World Health Organization / Data version: Globocan 2022 (version 1.1) – 08.02.20242.
Risk Factors
Helicobacter pylori Infection
H. pylori infection is the primary risk factor, with an estimated regional prevalence of 65-80% based on recent epidemiological studies. In rural areas of countries such as Peru, Bolivia, and Mexico, this prevalence can reach 85-90%3,6,7.
CagA-positive H. pylori strains are associated with increased gastric cancer risk due to their ability to induce DNA double-stranded breaks and compromise DNA repair mechanisms, leading to genomic instability8. CagA-positive strains are particularly prevalent in Chile and Costa Rica (>70% of asymptomatic subjects), correlating with their high gastric cancer rates9.
Across LATAM, the prevalence of H. pylori infection is exacerbated by factors such as limited access to clean water and sanitation. Different studies indicate a strong association between low socioeconomic status and higher infection rates3.
Dietary Factors
High salt intake has been consistently associated with an increased risk of gastric cancer10. Adults in most Latin American countries exceed the 5g WHO sodium limit11, averaging about 8 g/day in Mexico and Argentina12,13, 9.3g in Brazil14, 9.4g in Chile, and nearly 12 g in Colombia15. Conversely, in Brazil and Mexico, lower consumption of antioxidant-rich fruits and vegetables in rural and low-income communities exacerbates the disease risk16,17,18.
Genetic and Hereditary Factors
Hereditary risk factors for gastric cancer are primarily associated with specific genetic syndromes. The most well-characterized hereditary syndrome is Hereditary Diffuse Gastric Cancer (HDGC), caused by germline mutations in the CDH1 gene, and less commonly in the CTNNA1 gene. Another hereditary syndrome is Gastric Adenocarcinoma and Proximal Polyposis of the Stomach (GAPPS), which is linked to germline mutations in promoter 1B of the APC gene. Gastric cancer is also a component of other hereditary cancer syndromes, including Lynch syndrome, Familial Adenomatous Polyposis, MUTYH-associated polyposis, Peutz-Jeghers syndrome, Juvenile Polyposis syndrome, Li-Fraumeni syndrome, Cowden syndrome, and Hereditary Breast and Ovarian Cancer syndrome19.
Hereditary diffuse gastric cancer, associated with CDH1 gene mutations, is rare, but a high prevalence of germline variants has been reported in Mexican and Hispanic/Latino populations. Wang et al. found germline CDH1 variants in 16 % cases, a four-fold enrichment over the non-Latino series and the highest rate reported for any ethnic group to date. Complementing these germline findings, Bustos-Carpinteyro et al. showed that Mexican diffuse and mixed tumours frequently silence CDH1 somatically: promoter hyper-methylation was present in 64 %-67 % of cases and loss of heterozygosity in 13 %. Together, these studies indicate that both inherited and acquired inactivation of CDH1 is a characteristic molecular hallmark of gastric cancers arising in Hispanic/Latino populations20,21.
Studies in Brazil, Costa Rica, Mexico, and Colombia have identified genetic polymorphisms linked to an increased risk of gastric cancer in individuals with a family history, particularly in high-incidence areas22. The following germline risk variants were associated with an increased risk of developing somatic gastric cancer: IL-4, IL-8, TNF-α, PTGS2, NFKB1, RAF1, KRAS, and MAPK1 in the Brazilian, IL-10 in the Chilean, IL-10 in the Colombian, EGFR and ERRB2 in the Mexican, TCF7L2 and Chr8q24 in the Venezuelan population23.
Other Factors
Smoking is a well-established risk factor for gastric cancer. Current smokers have a relative risk (RR) of 1.53 compared to never smokers, and former smokers have an RR of 1.30. The CARMELA study found that Santiago, Chile, had the highest smoking prevalence at 45.4%24.
Heavy alcohol consumption is also associated with an increased risk of gastric cancer. More than 4 drinks a day is associated with an Odds ratio (OR) of 1.26 compared to abstainers. The higher rates of alcohol consumption of LATAM countries are found in countries like Argentina, Brazil, and Ecuador25.
Limited Access to Healthcare
Besides the “classic” risk factors mentioned above, the healthcare infrastructure in LATAM often lacks the resources and capacity to effectively manage and treat H. pylori infections and screen for hereditary cases. This is compounded by limited access to healthcare services and a shortage of healthcare professionals, which can delay diagnosis and treatment26.
Clinical Presentation and Diagnosis
Demographics
Gastric cancer often presents at a younger age in Hispanic populations compared to non-Hispanic populations. A significant proportion of cases occur before the age of 40, with studies reporting a frequency of 15% in Mexico and 13% in Colombia for patients diagnosed under 40 years old27,28,29,30. A cohort study encompassing countries in LATAM and Europe (EU) between 2018 and 2019 found that the median age at diagnosis was notably younger in LATAM (63 years) compared to Europe (70 years). A slight male predominance is observed, consistent with findings in other regions. A high proportion of LATAM patients are diagnosed at advanced stages. In Colombia, 55% of cases were diagnosed in stage IV31. In Mexico, this figure was 72%32,33. Across LATAM, stage IV diagnoses account for 44.8% of cases34, and in Chile, 56.1% of patients present with advanced disease35.
Regarding histological subtypes based on Lauren’s classification, the diffuse subtype was significantly more frequent in LATAM (44.9%) compared to EU populations (21.3%)34. In a Mexican cohort, the diffuse subtype predominated in 55.2%, followed by intestinal in 28.2%, and undifferentiated in 6%. Among diffuse cases, 72% exhibited signet-ring cells36. Signet-ring-cell prevalence shows striking geographic heterogeneity. In Mexico, large contemporary series place the frequency at the upper end of the global spectrum: signet-ring morphology was documented in 68.9 % of 956 stage-IV patients treated at a national referral center37. Across Latin America, the LEGACY study found a signet-ring component in 38 % of cases, significantly higher than the 27 % reported in parallel European cohorts38. Outside the region, the proportion falls: population-based U.S. estimates range from 8 % to 30 %39, while long-running Japanese surgical series record only ≈ 3-4 %40. These data underline how Latin-American patients carry a disproportionately high burden of signet-ring-cell gastric cancer.
Symptoms
In its early stages, gastric cancer symptoms are often nonspecific and may be mistaken for acid peptic disease. Unfortunately, the presence of symptoms in gastric cancer is frequently associated with advanced disease. Common symptoms at diagnosis include abdominal pain, weight loss, early satiety, nausea, vomiting, dyspepsia, and gastrointestinal bleeding, frequently reported in emergency settings. In Mexico, 98% of patients presented with symptoms at diagnosis, with 72.6% already in stage IV32. Similarly, in Colombia, the most frequent symptoms were dyspepsia (60.6%), weight loss (43.6%), and abdominal pain (41.7%)30. Dysphagia emerged as the predominant symptom in the EU and LATAM cohorts, affecting 42.4% of patients. The Gastro Esophageal Junction (GEJ) was the primary tumor site associated with dysphagia, occurring in 66.7% of cases34.
Diagnosis and Clinical Staging
Endoscopy is the most commonly used method for obtaining a histopathological diagnosis. While for staging, computed tomography (CT) is the most frequently employed method in LATAM. Additionally, 6.9% of LATAM patients underwent PET/CT imaging, and 68.2% underwent endoscopic ultrasound (EUS) staging34.
Delays in diagnosis and treatment initiation are common in LATAM. For instance, at a reference center in Mexico, the median time from symptom onset to histopathological diagnosis was 4.21 months (IQR 2.25–8.48), and the time from symptom onset to the initiation of oncological treatment of 5.28 months (IQR 2.86–9.52)32. In a high-risk population of Chile, this time was reported of more than 3 months35.
The most common metastatic sites in LATAM include the peritoneum, liver, retroperitoneum, ovaries, and lungs, reflecting the aggressive biology of this disease34,41. In the Mexican population, metastases frequently involve the peritoneum (76.1%), non-regional lymph nodes (38.9%), liver (22.8%), lung (26.7%), bones (9.4%), and ovaries in female patients (12.8%). At diagnosis, morbidity associated with peritoneal disease is present in up to 30% of patients. Peritoneal disease is associated with reduced treatment rates and poorer prognosis. Additionally, primary tumor-related morbidity is observed in 68% of patients, with obstruction symptoms in 56%, and bleeding in 27%41.
Table 1. Clinical and demographic characteristics ad diagnosis of patients with gastric cancer among different countries of Latin America
| Novick, D.Argentina, N=10142 | Brito, ABC. Brazil, N=1,406 43 | Bravo, LE.Colombia, N=8,549 31 | Mantilla, MJ. Colombia, N=259 30 | Heise, K. Chile, N=529 35 | Hernández-Félix, JH. Mexico, N=270 32 | Ruiz, CG. Mexico, N=1,130 33 | Acuña, S. Ecuador, N=159 44 | Freile, LATAM cohort 34 | |
|---|---|---|---|---|---|---|---|---|---|
| Age | 57.7 (+10.9) Mean, SD | 61.5 Mean, SD NR | 65 (54-74) Median, IQR | 62 (49-73) Median, IQR | NR | 58 (25-75) Median, IQR | 55.8 (+- 13.6) Mean, SD | 61 (15-91) Median, min and max | 63 (53-71) Median, IQR |
| <40 years (%) | NR | NR | 11.2 | 13.9 | NR | 13.7 | 17.5 | NR | NR |
| Sex (%) Men Woman | - 79.2 20.8 | - 60.7 39.3 | - 61.1 38.9 | - 57.3 42.7 | - 69 31 | - 46.7 53.3 | - 56 44 | - 47 53 | - 59 41 |
| Clinical Stage (%) | I - 3 II - 4 III - 5 IV - 41.5 Unk - 46.5 | I - 16.7 II - 16.9 III - 18.3 IV - 38.9 Unk - 9.1 | Early - 16.9 Advanced - 55.1 Unk - 28 | NR | I - 1.7 II - 3.8 III - 10 IV - 56.1 Unk - 28.4 | I - 4.8 II - 8.3 III - 14.3 IV - 72.6 | I - 2.8 II - 2.9 III - 26.3 IV - 68 | I - 6 II - 12 III - 35 IV - 37 Unk - 10 | I-II - 16.5 III - 38.7 IV - 44.8 |
| Lauren classification (%) Intestinal Diffuse Mixed Undetermined Unknown Other | - - - 17.8 26.7 0 3 38.6 13.9 | - - - 39.76 33.93 NR 24.32 0 1.99 | NR | - - - 53.8 38.9 7.3 NR NR NA | - - - 59.1 40.9 NA NA NA NA | - - - 21.6 53.9 3.3 20.8 0.4 NA | - - - 30 54 5 11 NA NA | NR | - - - 46.3 44.9 8.8 NA NA NA |
Abbreviations: IQR: Interquartile range. NR: Not Reported. NA: Not Available. Unk: Unknown. SD: Standard Deviation.
Molecular Biomarkers in the Latin American Population
Current clinical practice guidelines for the treatment of gastric cancer recommend immunohistochemistry (IHC) and/or molecular testing for HER2/ERB2 status, microsatellite instability (MSI) or mismatch repair (MMR) status, PD-L1 (programmed Death-ligand 1) expression, and CLDN (Claudin) 18.2, as these biomarkers influence the clinical management of advanced gastric cancer. Additional molecular alterations with potential targeted therapies include high tumor mutational burden (TMB-H), NTRK gene fusions, RET gene fusions, and BRAF V600E mutations. The availability of biomarker testing continues to grow, defining new treatment strategies45.
Access to biomarker testing remains a challenge in LATAM, hindered by availability, cost, and the specialized training required by pathologists. In many LATAM countries, biomarker testing is sponsored by the pharmaceutical industry. Limited data is available regarding the molecular biomarker status of gastric cancer in LATAM populations.
MSI-high or deficiency in MMR (dMMR) is one of the best predictive biomarkers of response to immune checkpoint blockade (ICB)45. MSI/dMMR has been reported in LATAM patients with gastric cancer at a frequency ranging from 5% to 20.9%46,47,48,49,50,51,52. The highest frequency (20.9%) was reported in the Brazilian population in a cohort of patients exclusively with resectable gastric cancer47. Outside Latin America, the proportion of dMMR/MSI-high gastric cancers shows marked geographic variation. Western European cohorts report rates around 12 %, but figures climb to ≈ 24 % in an Italian high-risk region53,54, whereas population-based studies from North America average 9-10 %55. In East Asia, prevalence is generally lower, about 8–11 % in Korea and China54,56 and only 3-5 % in Japanese series restricted to advanced disease, while Middle-Eastern data (Iran) are near 7 %56. These contrasts highlight both true biological heterogeneity and the influence of stage distribution and testing algorithms, reinforcing the need for region-specific screening strategies when considering immunotherapy or adjuvant-therapy de-escalation in dMMR/MSI-H disease.
Epstein-Barr Virus (EBV) associated gastric cancer is a distinct molecular subtype with a favorable prognosis57. Additionally, it has been reported as a predictive biomarker of response to ICB58,59. In the LATAM population, EBV positivity among patients with gastric cancer has been reported in 7 to 22%46,52,60,61,62,63,64,65,66. However, the traditional method to detect EBV (EBV-encoded RNA “EBER” in situ hybridization, ISH) could cause false-positive/false-negative results, and better methods (next-generation sequencing, NGS) to detect EBV-associated gastric cancer are recommended58.
HER/ERB2 overexpression in the LATAM population has been reported with less frequency compared to other populations45. The lowest frequency was reported in a cohort of Mexican patients (3.7%), followed by a Colombian cohort (5%)48,67. The overexpression of HER2 among LATAM patients with gastric cancer is between 3.7-15.8%42,48,52,67,68,69,70,71,72,73,74. Outside Latin America, HER2-positive gastric cancer accounts for around one-fifth of cases in Western series (~20 % in Europe/USA)75, rises slightly in Japan to ~21 %76, but is less frequent across East Asia, averaging 10-12 % in China77,78. HER2 status is an important biomarker in gastric cancer as it has therapeutic implications, with available targeted therapy that improves prognosis in this group of patients45.
PD-L1 positivity expression by IHC is also used as a predictive biomarker of response to ICB79,80. However, in advanced gastric cancer, different score systems have been adopted to predict response to ICB, and there is no consensus regarding the assessment criteria of PD-L1 staining and the best PD-L1 score predictive system81. Information regarding PD-L1 expression in LATAM patients with gastric cancer is scarce. In a cohort of patients with gastric cancer in Argentina, PD-L1 was positive in tumor cells and in infiltrating immune cells in 28%46. In Brazilian patients with advanced gastric cancer, the Combined Positive Score (CPS) PD-L1 1 was present in 15.8% of patients, while the Tumor Proportion Score (TPS) PD-L1 1 in 12%82. According to the Force1 study, in Chilean patients with gastric cancer, the frequency of CPS PD-L1 10 is 28.9%52. In Colombia, the CPS PD-L1 1 was positive in 30.6%, and 5 in 22.7% of patients with advanced gastric cancer48. Large international series show that PD-L1 positivity (CPS ≥ 1) runs around 40-65 % of gastric cancers worldwide, with Western and East-Asian cohorts occupying similar parts of that range, so LATAM reported prevalences are indeed lower than those reported elsewhere83.
Across Brazil, Mexico, and Chile, biomarker distributions differ in clinically meaningful ways (e.g., higher dMMR in Brazil’s resected cohort; higher HER2 in Chile). PD-L1 estimates vary widely owing to CPS/TPS scoring, antibody platforms, tumor stage, and sampling. These differences, together with heterogeneous access to testing and therapies across public systems, shape country-level eligibility for targeted and IO-based regimens. Relevant information on molecular biomarkers in patients with gastric cancer from LATAM countries is summarized in Table 2.
Table 2. Frequency of different molecular biomarkers in patients with gastric cancer from LATAM countries
| Molecular Biomarker Frequency | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Country | dMMR/MSI%38,46,47,48,49, 50,51,52 | EBV % 38,46,52,60,61,62,63, 64,65,66 | HER2/ERB2 positive %38,42,48,52,67,68, 70,71,72,73,74,75 | PD-L1 % 38,46,49, 52,83 | TMB high >8 mut/mb %52 | ||||
| Argentina | 8 | 21 | 8.7 | 28 | NA | ||||
| Brazil | 20.9 | 6.9-10.5 | 1.7-5.9 | CPS 1:15.8 TPS 1:12 | NA | ||||
| Chile | 13 | 13-22 | 10-13.3 | CPS 10:28.9 | 20.79 | ||||
| Colombia | 5-12.2 | 13-21.4 | 5 | CPS ≥1:30.6 CPS 5:22.7 | NA | ||||
| Guatemala | NA | 21.1 | NA | NA | NA | ||||
| Mexico | 8.3-13.5 | 7.3 | 3.7-15.8 | NA | NA | ||||
| Peru | NA | 8.4 | 9 | NA | NA | ||||
| LATAM cohort | 10 | 7 | 4 | CPS 1:69 CPS ≥5:48 CPS ≥10:40 | NA | ||||
Abbreviations: CPS: Combined Positive Score. dMMR: deficiency in Mismatch Repair. ERB2: Erythroblastic Oncogene B. EVB: Epstein-Barr Virus. HER2: Human Epidermal Growth Factor Receptor 2. Mb: Megabase. MSI: Microsatellite Instability. Mut: Mutations. NA: Not Available. PD-L1: Programmed Death Ligand 1. TMB: Tumor Mutational Burden.
Prognosis
Survival outcomes for patients with gastric cancer in LATAM are generally poor. In México, data from a retrospective series at a reference center report an estimated 5-year OS of 85 % for stage I, 58 % for stage II, 18% for stage III, and 3 % for stage IV32. In Chile, the 5-year survival rate for advanced gastric cancer is only 12.3%35. In Colombia, according to an analysis of a national cancer registry, the 3-year survival rate for patients with gastric cancer, independent of clinical stage, is 36.8% (95% CI: 35.5-38.1). With better survival for those diagnosed at early stages (I-II) 58.1% (95% CI: 54.5-61.5), compared with those at advanced stages 25.1% (95% CI: 23.4-26.9; p<0.001)31.
Across Latin America, the overall 5-year survival rate for gastric cancer is approximately 7%34. This poor survival probably reflects the aggressiveness of the disease. Patients with gastric cancer in LATAM present more frequently with poor prognosis histology (more diffuse compared to intestinal subtype), more signet ring cell component, and more peritoneal disease with morbidity that commonly precludes treatment (see clinical characteristics of LATAM population in table 1)30,32.
In Northeastern Brazil, younger patients with distal gastric cancer often present with aggressive, diffuse, and poorly differentiated tumors, frequently at advanced stages. This aggressive presentation contributes to poor survival outcomes, largely due to late diagnosis rather than age alone84. Similarly, in rural Western Honduras, late-stage presentation with pyloric obstruction is common. Survival rates in this region are exceptionally low, with a median survival time of just 4.8 months, highlighting the impact of limited access to treatment and late-stage diagnosis85.
Despite these statistics, a declining trend in gastric cancer mortality has been observed over the past 25 years in some countries. Díaz et al. show that age-standardized gastric-cancer mortality in Chile fell from 28.5 to 15.0 per 100,000 in men and 11.0 to 5.5 in women, while Argentina dropped from 24.8 to 15.5 (men) and 8.6 to 6.0 (women) between 1990 and 2015, roughly a 30–50 % reduction. This improvement is likely attributed to enhanced healthcare systems and the implementation of early detection strategies86.
Standard Treatment and Real-world Data in Latin America
As in other regions of the world, the standard treatment for gastric cancer in LATAM requires a comprehensive approach that involves surgery, chemotherapy, radiotherapy, targeted therapy, and immunotherapy, tailored to clinical stage and molecular subtype. However, access to optimal treatment in LATAM is heterogeneous and challenging. In many countries, healthcare services are centralized, leading to long waiting times for referrals to specialized oncology services. Additionally, access to innovative therapies, such as targeted therapy and immunotherapy, remains limited in the public healthcare systems.
Treatment in the Early Stages
Surgery is the cornerstone of treatment for early-stage gastric cancer. Many countries in LATAM have established specialized surgical centers to improve outcomes, including reduced morbidity and mortality and better oncological results. These centers often aim to perform D2 lymphadenectomy, with some offering minimally invasive approaches, such as laparoscopy, and, less frequently, robotic surgery. Argentina, Brazil, Chile, Colombia, Mexico, and Peru have specialized public reference centers. Unfortunately, despite these advancements, surgical management of gastric cancer is still performed by general surgeons in some regions of LATAM, highlighting disparities in access to specialized care87.
For resectable locally advanced disease, both perioperative and adjuvant chemotherapy approaches are commonly employed in specialized centers. The perioperative FLOT chemotherapy regimen has gained traction in recent years for pMMR tumors88,89. According to Freile et al, surgery was performed with curative intent in 48.9% of patients in a LATAM cohort, while 12% underwent surgery for palliative purposes. Systemic treatment with curative intent was initiated in 46.4% of patients. Additionally, neoadjuvant chemoradiotherapy was administered to 67% of patients with stage III GEJ cancer34.
Recently, perioperative immunotherapy has been added to clinical practice guidelines recommendations for patients with locally advanced gastric cancer and MSI/dMMR45. However, there is currently no real-world data on the use of perioperative immunotherapy for locally advanced gastric cancer with dMMR in LATAM, nor information regarding its approval for regulatory agencies, likely indicating its use only in clinical trials.
Treatment in advanced disease
For advanced gastric cancer, chemotherapy remains the mainstay of treatment in LATAM. Common first-line regimens include doublet or triplet therapies based on a platinum-fluoropyrimidine backbone, with the addition of a third drug in selected cases. In the past, epirubicin was added as the third drug42; more recently, docetaxel is preferred. According to Freile, B. et al, chemotherapy regimens FLOT, FOLFOX, and CAPOX were used in 84.8% of patients with gastric cancer in LATAM34.
However, delayed access to health services results in many patients being unable to receive treatment. For example, a Mexican retrospective series reported that less than half of the patients with advanced gastric cancer received systemic first-line chemotherapy90. Additionally, only 14.4% of patients in LATAM received second-line treatment, and 3% received a third-line option34. Paclitaxel and irinotecan are common second and third-line options used.
In recent years, the addition of immunotherapy to chemotherapy has been recommended for the treatment of patients with advanced gastric cancer, as it improves survival of patients45,80,84. The addition of either nivolumab or pembrolizumab has been approved by different health regulatory agencies using different CPS PD-L1 cut-off points45. Most of the LATAM countries approved the use of nivolumab for those with a CPS PD-L1 expression of 5, with some exceptions. In Mexico, nivolumab is approved for all patients with advanced gastric cancer regardless of PD-L1 expression. Nevertheless, even though immunotherapy is approved, it does not translate into availability in all public institutions of LATAM, as it was reported on a real-world data registry where immunotherapy was only accessible in 12% of patients34.
For patients with dMMR/MSI tumors, clinical guidelines recommend immunotherapy as the first-line treatment, independent of PD-L1 status. Different drugs and regimens are recommended: dostarlimab, nivolumab, pembrolizumab, nivolumab plus ipilimumab, or the combination of chemotherapy with either nivolumab or pembrolizumab45. The phase 3 clinical trial CheckMate-649 showed an unprecedented advantage for the combination of ipilimumab plus nivolumab in patients with dMMR/MSI advanced gastric cancer, with a mOS Not Reached compared to 10 months with chemotherapy alone (HR 0.28, 95% CI 0.02-0.92)91. Other reports have confirmed the advantage of immunotherapy over chemotherapy alone, or combined with chemotherapy, for this population92. The use of immunotherapy for this indication is not approved by the health regulatory agencies of most countries in LATAM.
In patients with advanced gastric cancer and HER2 overexpression, clinical guidelines recommend the use of chemotherapy combined with the anti-HER2 therapy trastuzumab45. The addition of trastuzumab showed an improvement in OS in the TOGA trial93. Combining anti-HER2-targeted therapy with immunotherapy is a novel approach for this population and is also recommended in clinical guidelines45. The KEYNOTE-811 showed an improvement in objective response, progression-free survival (PFS), and OS with the combination of chemotherapy, trastuzumab, and pembrolizumab in patients with HER2-positive and CPS PD-L1 ≥194. There is no real-world data regarding the efficacy of combining chemotherapy, trastuzumab, and immunotherapy in LATAM. Yet, trastuzumab is available in most of the public institutions of the region. Pembrolizumab added to chemotherapy and trastuzumab is approved in some countries of LATAM (Ex, Mexico, Colombia).
New targeted therapies are emerging for the treatment of advanced gastric cancer. Claudin (CLDN) 18.2 and FGFR (Fibroblast Growth Factor Receptor) are new biomarkers with target drugs that have shown positive results in clinical trials. Zolbetuximab, a chimeric IgG1 antibody targeting CLDN18.2, showed significant PFS and OS benefit when combined with chemotherapy in the SPOTLIGHT and GLOW trials for CLDN18.2-positive, HER2-negative, previously untreated locally advanced unresectable or metastatic gastric adenocarcinoma patients95,96. Zolbetuximab is now recommended in clinical practice guidelines; however, approvals are still pending across LATAM, and the drug is not yet available45.
Bemarituzumab, a monoclonal antibody targeting FGFR2b, demonstrated improved PFS and OS in the phase II FIGHT trial when combined with chemotherapy in the first-line setting, particularly in patients with FGFR2b overexpression (IHC score 2+ or 3+ in ≥10% of tumor cells)97. FORTITUTE-101 is an ongoing phase 3 clinical trial that is evaluating the combination of bemarituzumab plus chemotherapy or placebo in the first-line setting of patients with advanced gastric cancer and overexpression of FGFR2b. The recruitment was completed, and the results are pending. The trial included patients from different LATAM countries: Argentina, Brazil, Chile, Colombia, Mexico, and Peru (NCT05052801). Bemarituzumab for advanced gastric cancer is not yet approved in any country.
Regarding antiangiogenic therapy, Ramucirumab showed limited efficacy as monotherapy (REGARD) but improved OS modestly when combined with paclitaxel in the RAINBOW phase 3 trial, establishing it as a second-line standard treatment45,98,99. However, Ramucirumab is not approved across all LATAM countries (i.e., Mexico), and where it is approved is not always available in public institutions.
Finally, even though clinical trials are strongly recommended by international guidelines, they are not commonly available in LATAM, and only a small proportion of our patients (1.1%) are enrolled34, reflecting significant gaps in research infrastructure and access.
Challenges and Availability in Latin America
Gastric cancer in LATAM demonstrates significant regional disparities, reflecting the heterogeneity in socioeconomic status, healthcare access, dietary habits, and environmental factors across the continent. These disparities influence the incidence, age at diagnosis, histological subtypes, diagnostic practices, and survival outcomes observed in different countries and regions.
Challenges and Availability of Diagnostic Tools
One of the key challenges in addressing gastric cancer in LATAM is the limited availability of diagnostic tools, particularly in rural and underserved areas. Endoscopy, a critical tool for the early detection and diagnosis of gastric cancer, is not uniformly accessible across the region. Human resource shortages further compound the problem. The number of gastroenterologists and trained endoscopic technicians is disproportionately low in rural and low-income areas. For instance, some regions report only a handful of specialists serving entire provinces, forcing patients to travel long distances to access care. This lack of trained professionals not only delays diagnosis but also compromises the quality of care provided, particularly in complex cases requiring advanced techniques such as endoscopic ultrasound (EUS) for staging100.
Absence of Advanced Treatments
The limited availability of advanced treatments such as chemotherapy, immunotherapy, and novel targeted therapies further exacerbates the disparities in gastric cancer outcomes. In many low- and middle-income countries in LATAM, standard chemotherapy regimens are often the only treatment option available. Access to immunotherapy agents, such as checkpoint inhibitors targeting PD-1/PD-L1, remains restricted due to high costs and limited healthcare budgets101,102. As a result, patients who could potentially benefit from these innovative treatments are left with suboptimal options.
Emerging therapies, such as Claudin inhibitors, which show promise in treating specific subtypes of gastric cancer, are virtually nonexistent in the region. These therapies, currently in clinical trials or early adoption stages in high-income countries, are years away from being accessible to LATAM populations. The lack of infrastructure for clinical trials and the high cost of importing these therapies create significant barriers to their introduction103,104,105. Consequently, patients are unable to access timely, cutting-edge treatments that could improve survival and quality of life.
Ongoing Clinical Trials in LATAM
Some of the ongoing clinical trials in the region LATAM are presented in Table 3.
Table 3. Ongoing clinical trials in LATAM evaluating systemic therapies for gastric and gastroesophageal junction cancers
| Clinical trial | Phase | Drugs/Intervention | Clinical setting | Status (LATAM) |
|---|---|---|---|---|
| NCT05152147 | III | Zanidatamab + chemotherapy ± tislelizumab | HER2-positive advanced/metastatic gastric or esophageal cancer | Active, not recruiting |
| NCT05052801 | III | Bemarituzumab or placebo + chemotherapy | FGFR2b overexpressed gastric cancer | Active, not recruiting |
| NCT05111626 | III | Bemarituzumab + chemotherapy + nivolumab vs chemotherapy + nivolumab | Untreated FGFR2b overexpressed advanced gastric/GEJ cancer | Active, not recruiting |
| NCT06356311 | II/III* | Sacituzumab tirumotecan (MK-2870) | Third-line advanced/metastatic gastroesophageal adenocarcinoma | Recruiting |
| NCT06731478 | III | Trastuzumab deruxtecan (TDXd) + chemotherapy + pembrolizumab + trastuzumab | First-line metastatic HER2-positive gastric/GEJ cancer | Recruiting |
| NCT06532006 | III | HLX22 + trastuzumab + chemotherapy | Advanced gastric/GEJ cancer | Recruiting |
| NCT06764875 | III | Rilvegostomig + fluoropyrimidine + trastuzumab deruxtecan | First-line HER2-positive gastric cancer | Recruiting |
| NCT06469944 | II/III* | Sacituzumab tirumotecan (MK-2870) + pembrolizumab + chemotherapy | First-line locally advanced unresectable/metastatic gastroesophageal adenocarcinoma | Recruiting |
Conclusion
Gastric cancer in Latin America (LATAM) is characterized by marked heterogeneity in incidence, stage at diagnosis, biomarker distributions (HER2, MSI/dMMR, EBV, PD-L1), and access to diagnostics and therapies. These differences shape eligibility for biomarker-directed and immuno-oncology regimens and contribute to persistently poor outcomes in many settings, even as some countries show encouraging mortality declines. Closing diagnostic and treatment gaps is an urgent public-health and health-systems priority, with steps available now that can improve care while building regional capacity.
For policymakers and public health, the most immediate opportunities are to implement targeted H. pylori test-and-treat strategies in high-risk subregions; to subsidize and standardize a core biomarker panel (HER2 by IHC/ISH, MSI/dMMR, PD-L1 using CPS, and CLDN18.2) with centralized quality assurance; to invest in endoscopy/EUS, pathology (including reflex biomarker workflows), and referral pathways that shorten time to diagnosis; and to create evidence-based access pathways for immunotherapy and targeted agents through health-technology assessment and, where appropriate, risk-sharing agreements.
For clinicians and health systems, delivering guideline-concordant perioperative care, harmonizing pathology and biomarker reporting (specimen adequacy, HER2 algorithms, MSI methods, PD-L1 CPS), and re-testing when clinically indicated can immediately raise the standard of care. Early referral to multidisciplinary GI teams and clinical-trial centers, paired with patient navigation to reduce loss-to-follow-up, should be routine elements of care pathways, alongside timely nutritional and palliative support.
For researchers and trial networks, expanding population-based registries that capture stage, biomarkers, treatments, and outcomes with harmonized data dictionaries will enable cross-country comparisons and quality improvement. Increasing LATAM participation in practice-shaping trials, including pragmatic designs and biomarker-enriched neoadjuvant and adjuvant studies, and developing regional basket and umbrella platforms are practical ways to accelerate evidence generation. Implementation science, health-economics evaluations of H. pylori programs and endoscopy access, and real-world studies of immunotherapy and targeted strategies are equally essential to guide policy and practice.
With coordinated action across policy, clinical care, and research, LATAM can achieve earlier diagnosis, broader biomarker-driven treatment, and tangible survival gains, narrowing global disparities in gastric cancer outcomes.
Competing Interests: The authors declare no competing financial interests.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
License
© Author(s) 2025.
This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, and unrestricted adaptation and reuse, including for commercial purposes, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
To view a copy of this license, visit https://creativecommons.org/licenses/by/4.0/.
References
-
Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024 Jan–Feb;74(1):12–49.
-
Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024 May–Jun;74(3):229–63.
-
Porras C, Nodora J, Sexton R, et al. Epidemiology of Helicobacter pylori infection in six Latin American countries (SWOG Trial S0701). Cancer Causes Control. 2013 Feb;24(2):209–15.
-
González-Pons M, Soto-Salgado M, Sevilla J, et al. Seroprevalence of Helicobacter pylori in Hispanics living in Puerto Rico: a population-based study. Helicobacter. 2018 Feb;23(1):e12453.
-
Banydeen R, Rose AM, Martin D, et al. Advancing cancer control through research and cancer registry collaborations in the Caribbean. Cancer Control. 2015 Oct;22(4):520–30.
-
Torres J, Lopez L, Lazcano E, et al. Trends in Helicobacter pylori infection and gastric cancer in Mexico. Cancer Epidemiol Biomarkers Prev. 2005 Aug;14(8):1874–7.
-
Alvarado-Esquivel C. Seroepidemiology of Helicobacter pylori infection in pregnant women in rural Durango, Mexico. Int J Biomed Sci. 2013;9(4):224–9.
-
Takahashi-Kanemitsu A, Knight CT, Hatakeyama M. Molecular anatomy and pathogenic actions of Helicobacter pylori CagA that underpin gastric carcinogenesis. Cell Mol Immunol. 2020 Jan;17(1):50–63.
-
Con SA, Takeuchi H, Valerín AL, et al. Diversity of Helicobacter pylori cagA and vacA genes in Costa Rica: its relationship with atrophic gastritis and gastric cancer. Helicobacter. 2007 Oct;12(5):547–52.
-
Wu X, Chen L, Cheng J, et al. Effect of dietary salt intake on risk of gastric cancer: a systematic review and meta-analysis of case-control studies. Nutrients. 2022 Oct 12;14(20):4260.
-
Pan American Health Organization. Salt intake [Internet]. [cited 2025 Jul 8]. Available from: https://www.paho.org/fr/node/90878
-
Guarnieri L, Castronuovo L, Flexner N, et al. Monitoring sodium content in processed and ultraprocessed foods in Argentina 2022: compliance with national legislation and regional targets. Public Health Nutr. 2024 Oct 2;27(1):e193.
-
Gutiérrez-Salmeán G, Miranda-Alatriste PV, Benítez-Alday P, et al. Knowledge, attitudes, and behaviors toward salt consumption and its association with 24-hour urinary sodium and potassium excretion in adults living in Mexico City: cross-sectional study. Interact J Med Res. 2024 Nov 18;13:e57265.
-
Mill JG, Malta DC, Nilson EAF, et al. Factors associated with salt intake in the Brazilian adult population: National Health Survey. Cien Saude Colet. 2021 Feb;26(2):555–67.
-
Margozzini P, Passi Á. Encuesta Nacional de Salud, ENS 2016–2017: un aporte a la planificación sanitaria y políticas públicas en Chile. ARS Med Rev Cienc Med. 2018;43(1):30–4.
-
Serafini M, Bellocco R, Wolk A, et al. Total antioxidant potential of fruit and vegetables and risk of gastric cancer. Gastroenterology. 2002 Oct;123(4):985–91.
-
Ramírez-Silva I, Rivera JA, Ponce X, et al. Fruit and vegetable intake in the Mexican population: results from the Mexican National Health and Nutrition Survey 2006. Salud Publica Mex. 2009;51(Suppl 4):S574–85.
-
Machado RHV, Feferbaum R, Leone C. Fruit intake and obesity: fruit and vegetables consumption and obesity in Brazil. J Hum Growth Dev. 2016;26(2):243–52.
-
Carneiro F. Familial and hereditary gastric cancer: an overview. Best Pract Res Clin Gastroenterol. 2022 Jun–Aug;58–59:101800.
-
Wang SC, Yeu Y, Hammer STG, et al. Hispanic/Latino patients with gastric adenocarcinoma have distinct molecular profiles, including a high rate of germline CDH1 variants. Cancer Res. 2020 Jun 1;80(11):2114–24.
-
Bustos-Carpinteyro AR, Oliveira C, Sousa A, et al. CDH1 somatic alterations in Mexican patients with diffuse and mixed sporadic gastric cancer. BMC Cancer. 2019 Jan 14;19(1):69.
-
Chiurillo MA. Role of gene polymorphisms in gastric cancer and its precursor lesions: current knowledge and perspectives in Latin American countries. World J Gastroenterol. 2014;20(16):4503–15.
-
Cerrato-Izaguirre D, Chirino YI, García-Cuellar CM, et al. Mutational landscape of gastric adenocarcinoma in Latin America: a genetic approach for precision medicine. Genes Dis. 2021;9(4):928–40.
-
Champagne BM, Sebrié EM, Schargrodsky H, et al. Tobacco smoking in seven Latin American cities: the CARMELA study. Tob Control. 2010;19(6):457–62.
-
Díaz LA, Idalsoaga F, Fuentes-López E, et al. Impact of public health policies on alcohol-associated liver disease in Latin America: an ecological multinational study. Hepatology. 2021 Nov;74(5):2478–90.
-
Coelho LG, Coelho MC. Clinical management of Helicobacter pylori: the Latin American perspective. Dig Dis. 2014;32(3):302–9.
-
Vitiello GA, Hani L, Wang A, et al. Clinical presentation patterns and survival outcomes of Hispanic patients with gastric cancer. J Surg Res. 2021;268:606–15.
-
Karalis JD, Ju MR, Mansour JC, et al. The presentation of Hispanic gastric cancer patients varies by location of patient ancestry. J Surg Oncol. 2021;124(7):1051–9.
-
Calderillo-Ruíz G, Díaz-Romero MC, Carbajal-López B, et al. Latin American young patients with gastric adenocarcinoma: worst prognosis and outcomes. J Gastrointest Oncol. 2023;14(5):2018–27.
-
Mantilla MJ, Chaves JJ, Ochoa-Vera M, et al. Clinical characteristics of early-onset gastric cancer: a study in a Colombian population. Rev Gastroenterol Peru. 2023;43(3):236–41.
-
Bravo LE, Hernández Vargas JA, Collazos P, et al. Survival in stomach cancer: analysis of a national cancer information system and a population-based cancer registry in Colombia. Colomb Med (Cali). 2022;53(4):e2025126.
-
Hernandez-Felix JH, Meneses-Medina MI, Jiménez Meza MA, et al. Clinical presentation and delay to diagnosis and treatment in Mexican patients with gastric cancer. J Clin Oncol. 2024;42(3 Suppl):291.
-
Ruiz GC, Reynoso N, Diaz C, et al. Epidemiological data of patients with gastric cancer in Mexico. Ann Oncol. 2012;23(Suppl 9):ix251.
-
Freile B, van Schooten TS, Derks S, et al. Gastric cancer hospital-based registry: real-world gastric cancer data from Latin America and Europe. ESMO Gastrointest Oncol. 2024;6:100088.
-
Heise K, Bertran E, Andia ME, et al. Incidence and survival of stomach cancer in a high-risk population of Chile. World J Gastroenterol. 2009;15(15):1854–62.
-
Martínez-Galindo MG, Zamarripa-Dorsey F, Carmona-Castañeda A, et al. Histopathologic characteristics of gastric adenocarcinoma in Mexican patients: a 10-year experience at the Hospital Juárez of Mexico. Rev Gastroenterol Mex. 2015;80(1):21–6.
-
León AM, Hall WB, Lino LS, et al. Identification of prognostic factors for survival in patients with metastatic gastric adenocarcinoma in a Mexican population. Rev Gastroenterol Mex (Engl Ed). 2024;89(3):340–6.
-
Martínez-Ciarpaglini C, Barros R, Caballero C, et al. Comprehensive histopathological analysis of gastric cancer in European and Latin American populations reveals differences in PDL1, HER2, p53 and MUC6 expression. Gastric Cancer. 2025;28(2):160–73.
-
French K, Jayasekera M, Gage J, et al. Incidental finding of gastric signet ring cell carcinoma: a case report. Am J Gastroenterol. 2022;117(10 Suppl):e2273.
-
Maehara Y, Sakaguchi Y, Moriguchi S, et al. Signet ring cell carcinoma of the stomach. Cancer. 1992;69(7):1645–50.
-
Meneses Medina MI, Valenzuela AK, Hernandez-Felix JH, et al. Morbidity associated to advanced gastric cancer and its impact on overall survival. J Clin Oncol. 2025;38(4 Suppl):327.
-
Novick D, Mendez G, Carballido M, et al. Retrospective analysis of patients with locally advanced or metastatic gastric cancer in Argentina. Medwave. 2019;19(8):e7692.
-
Brito ABC, Felismino TC, E Silva DRM, et al. Survival trends in gastric cancer in Brazil: real-life data from a large cancer center. Ecancermedicalscience. 2024;18:1706.
-
Acuña S, Solís P, Oñate P, et al. Epidemiología del cáncer de estómago en un centro de referencia en Ecuador. Rev Med Vozandes. 2020;31(2):19–25.
-
McMillian N, Stein M, Ajani JA, et al. NCCN Clinical Practice Guidelines in Oncology: gastric cancer. Version 5.2024 [Internet]. National Comprehensive Cancer Network; 2024 [cited 2025 Oct 4]. Available from: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1434
-
Kujaruk M, Salanova R, Ruiz G, et al. Molecular and pathological features in patients with gastric cancer in Argentina: association between Epstein–Barr virus, MSI, PD-L1 expression and T-cells infiltration. Ann Oncol. 2019;30(Suppl 4):iv85.
-
Ramos MFKP, Pereira MA, de Mello ES, et al. Gastric cancer molecular classification based on immunohistochemistry and in situ hybridization: analysis in western patients after curative-intent surgery. World J Clin Oncol. 2021;12(8):688–701.
-
Ballen DF, Ojeda K, Gualdron A, et al. Real-world prevalence of biomarkers for treatment of advanced gastric cancer or gastroesophageal junction cancer in a cohort of Colombian patients [Internet]. Instituto Nacional de Cancerología; 2025 [cited 2025 Oct 4]. Available from: https://repositorio.cancer.gov.co/items/bdaa2e7c-f9ef-4adc-b3da-5d7cedd04920
-
González-Motta A, Negrete-Tobar G, Messa-Botero OA, et al. Prevalence of microsatellite instability in gastric and gastroesophageal junction cancer patients from a Latin American country. Gac Med Mex. 2024;160(4):393–8.
-
Dorantes-Heredia R, Motola-Kuba D, Escamilla-López I, et al. Prevalence of mismatch repair deficiency in advanced solid tumors (colorectal cancer and non-colorectal cancer) in one Mexican institution. J Pers Med. 2024;14(12):1152.
-
Diaz Romero MC, Miyagui-Adame SM, Calderillo Ruiz G, et al. Impact of DNA mismatch repair status on prognosis of patients with locally advanced gastric cancer in a Mexican population. J Clin Oncol. 2024;42(3 Suppl):408.
-
Cordova-Delgado M, Pinto MP, Retamal IN, et al. High proportion of potential candidates for immunotherapy in a Chilean cohort of gastric cancer patients: results of the FORCE1 study. Cancers (Basel). 2019;11(9):1275.
-
Tran-Minh ML, Lehmann-Che J, Lambert J, et al. Prevalence and prognosis of microsatellite instability in oesogastric adenocarcinoma: NORDICAP 16-01. Clin Res Hepatol Gastroenterol. 2021;45(4):101691.
-
Wu H, Ma W, Jiang C, et al. Heterogeneity and adjuvant therapeutic approaches in MSI-H/dMMR resectable gastric cancer: emerging trends in immunotherapy. Ann Surg Oncol. 2023;30(13):8572–87.
-
Randon G, Aoki Y, Cohen R, et al. Outcomes and a prognostic classifier in patients with microsatellite instability-high metastatic gastric cancer receiving PD-1 blockade. J Immunother Cancer. 2023;11(6):e007104.
-
Talari FF, Bozorg A, Zeinali S, et al. Low incidence of microsatellite instability in gastric cancers and its association with clinicopathological characteristics: a comparative study. Sci Rep. 2023;13(1):21743.
-
Liu X, Liu J, Qiu H, et al. Prognostic significance of Epstein–Barr virus infection in gastric cancer: a meta-analysis. BMC Cancer. 2015;15:782.
-
Bai Y, Xie T, Wang Z, et al. Efficacy and predictive biomarkers of immunotherapy in Epstein–Barr virus-associated gastric cancer. J Immunother Cancer. 2022;10(3):e004080.
-
Xie T, Liu Y, Zhang Z, et al. Positive status of Epstein–Barr virus as a biomarker for gastric cancer immunotherapy: a prospective observational study. J Immunother. 2020;43(4):139–44.
-
de Lima MA, Ferreira MV, Barros MA, et al. Epstein–Barr virus-associated gastric carcinoma in Brazil: comparison between in situ hybridization and polymerase chain reaction detection. Braz J Microbiol. 2012;43(1):393–404.
-
Reyes ME, Zanella L, Riquelme I, et al. Exploring the genetic diversity of Epstein–Barr virus among patients with gastric cancer in southern Chile. Int J Mol Sci. 2023;24(14):11276.
-
Carrascal E, Koriyama C, Akiba S, Tamayo O, Itoh T, Eizuru Y, et al. Epstein–Barr virus-associated gastric carcinoma in Cali, Colombia. Oncol Rep. 2003;10(4):1059–62.
-
Vidal-Realpe A, Dueñas-Cuellar RA, Niño-Castaño VE, et al. Clinical and pathologic characteristics of gastric adenocarcinoma associated with Epstein–Barr virus in a region with a high incidence of gastric cancer in Colombia. Rev Gastroenterol Mex (Engl Ed). 2023;88(3):256–66.
-
Lange KJ, Siliézar MM, López NY, et al. Identificación del virus Epstein–Barr por hibridación in situ en pacientes con cáncer gástrico que asisten al Instituto de Cancerología (Incan) de Guatemala. Cienc Tecnol Salud. 2020;7(1):00–00.
-
Herrera-Goepfert R, Akiba S, Koriyama C, et al. Epstein–Barr virus-associated gastric carcinoma: evidence of age-dependence among a Mexican population. World J Gastroenterol. 2005 Oct 21;11(39):6096–103.
-
Beltrán Gárate B, Camara A, Kapsoli Sánchez MDC, et al. Impacto del virus Epstein–Barr en el cáncer gástrico en el Perú [Impact of the Epstein–Barr virus on gastric cancer in Peru]. Rev Gastroenterol Peru. 2019 Oct–Dec;39(4):319–22.
-
Cruz-Reyes C, Gamboa-Dominguez A. HER2 amplification in gastric cancer is a rare event restricted to the intestinal phenotype. Int J Surg Pathol. 2013;21(3):240–6.
-
Jung JE, Sérgio I, Ioshii O. Immunohistochemical assessment of HER2 expression in gastric cancer in a cohort of 118 Brazilian patients. J Bras Patol Med Lab. 2013;49(5):00–00.
-
Roa I, Slater J, Carvajal D, et al. Expresión y amplificación del gen HER2 en el cáncer gástrico avanzado [HER2 gene amplification and overexpression in advanced gastric cancer]. Rev Med Chil. 2013;141(11):1411–9.
-
Painemal C, Berrios M, Gallegos I, et al. Determination of the HER2 expression in Chilean patients with gastric cancer. Ann Oncol. 2012;23(Suppl 4):iv50.
-
López-Jiménez P, Tenorio-Torres JA, Villegas-Carlos F, et al. Prevalence of HER2 overexpression in gastric cancer at the National Medical Center 20 de Noviembre. Rev Esp Med Quir. 2018;23(1):5–9.
-
Alvarado-Cabrero I, Gil-Hernández S, Ruelas-Perea A, et al. Evaluación por inmunohistoquímica de la expresión del HER2 en cáncer gástrico: estudio clínico-patológico de 93 casos [Immunohistochemical assessment of HER2 expression in gastric cancer: a clinicopathologic study of 93 cases]. Cir Cir. 2017;85(6):504–9.
-
Diaz C, García-García E, Miyagui Adame S, et al. HER2 positive as the main biomarker for overall survival of advanced gastric adenocarcinoma: Latin American reality and challenges. Ann Oncol. 2023;34(Suppl 1):S67–8.
-
Beltrán Gárate B, Yabar Berrocal A. Expresión de HER2 en cáncer gástrico en el Perú [HER2 expression in gastric cancer in Peru]. Rev Gastroenterol Peru. 2010;30(4):324–7.
-
Janjigian YY, Werner D, Pauligk C, et al. Prognosis of metastatic gastric and gastroesophageal junction cancer by HER2 status: a European and USA international collaborative analysis. Ann Oncol. 2012;23(10):2656–62.
-
Matsusaka S, Nashimoto A, Nishikawa K, et al. Clinicopathological factors associated with HER2 status in gastric cancer: results from a prospective multicenter observational cohort study in a Japanese population (JFMC44-1101). Gastric Cancer. 2016;19(3):839–51.
-
Huang D, Li ZS, Fan XS, et al. [HER2 status in gastric adenocarcinoma of Chinese: a multicenter study of 40,842 patients]. Zhonghua Bing Li Xue Za Zhi. 2018;47(11):822–6.
-
Seo KW, Jeon T, Kim S, et al. Epidemiologic study of human epidermal growth factor receptor 2 expression in advanced/metastatic gastric cancer: an assessment of HER2 status in tumor tissue samples of gastric and gastroesophageal junction cancer. J Gastric Cancer. 2017;17(1):52–62.
-
Janjigian YY, Shitara K, Moehler M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398(10294):27–40.
-
Rha SY, Oh DY, Yañez P, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for HER2-negative advanced gastric cancer (KEYNOTE-859): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2023;24(11):1181–95.
-
Yamashita K, Iwatsuki M, Harada K, et al. Prognostic impacts of the combined positive score and the tumor proportion score for programmed death ligand-1 expression by double immunohistochemical staining in patients with advanced gastric cancer. Gastric Cancer. 2020;23(1):95–104.
-
Pereira MA, Ramos MFKP, Dias AR, et al. Scoring systems for PD-L1 expression and their prognostic impact in patients with resectable gastric cancer. Virchows Arch. 2021;478(6):1039–48.
-
Janjigian YY, Ajani JA, Moehler M, et al. First-line nivolumab plus chemotherapy for advanced gastric, gastroesophageal junction, and esophageal adenocarcinoma: 3-year follow-up of the phase III CheckMate 649 trial. J Clin Oncol. 2024;42(17):2012–20.
-
Braga-Neto MB, Carneiro JG, de Castro Barbosa AM, et al. Clinical characteristics of distal gastric cancer in young adults from Northeastern Brazil. BMC Cancer. 2018;18(1):131.
-
Dominguez RL, Montalvan-Sanchez EE, Norwood DA, et al. Population-based study of gastric cancer survival and associations in rural western Honduras. Cancer Epidemiol Biomarkers Prev. 2024;33(12):1578–85.
-
Diaz MDP, Icaza G, Nuñez L, et al. Gastric cancer mortality trends in the Southern Cone: disentangling age, period and cohort patterns in Argentina and Chile. Sci Rep. 2020;10(1):1526.
-
Santos E. Current approaches to gastric cancer in Peru and Mexico. Transl Gastroenterol Hepatol. 2017;2:55.
-
Müller B, García C, Sola JA, et al. Perioperative chemotherapy in locally advanced gastric cancer in Chile: from evidence to daily practice. Ecancermedicalscience. 2021;15:1244.
-
Verduzco-Aguirre HC, Meneses Medina MI, Valenzuela AK, et al. Treatment modalities and oncological outcomes in Mexican patients with localized gastric cancer. J Clin Oncol. 2020;38(4 Suppl):319.
-
Valenzuela AK, Meneses Medina MI, Camargo VR, et al. Treatment modalities and oncological outcomes in a cohort of patients with advanced gastric cancer in Mexico. J Clin Oncol. 2019;37(15 Suppl):e15590.
-
Shitara K, Ajani JA, Moehler M, et al. Nivolumab plus chemotherapy or ipilimumab in gastro-oesophageal cancer. Nature. 2022;603(7903):942–8.
-
Chao J, Fuchs CS, Shitara K, et al. Assessment of pembrolizumab therapy for the treatment of microsatellite instability-high gastric or gastroesophageal junction cancer among patients in the KEYNOTE-059, KEYNOTE-061, and KEYNOTE-062 clinical trials. JAMA Oncol. 2021;7(6):895–902.
-
Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687–97.
-
Janjigian YY, Kawazoe A, Bai Y, et al. Pembrolizumab plus trastuzumab and chemotherapy for HER2-positive gastric or gastro-oesophageal junction adenocarcinoma: interim analyses from the phase 3 KEYNOTE-811 randomised placebo-controlled trial. Lancet. 2023;402(10418):2197–208.
-
Shah MA, Shitara K, Ajani JA, et al. Zolbetuximab plus CAPOX in CLDN18.2-positive gastric or gastroesophageal junction adenocarcinoma: the randomized, phase 3 GLOW trial. Nat Med. 2023;29(8):2133–41.
-
Shitara K, Lordick F, Bang YJ, et al. Zolbetuximab plus mFOLFOX6 in patients with CLDN18.2-positive, HER2-negative, untreated, locally advanced unresectable or metastatic gastric or gastro-oesophageal junction adenocarcinoma (SPOTLIGHT): a multicentre, randomised, double-blind, phase 3 trial. Lancet. 2023;401(10389):1655–68.
-
Wainberg ZA, Enzinger PC, Kang YK, et al. Bemarituzumab in patients with FGFR2b-selected gastric or gastro-oesophageal junction adenocarcinoma (FIGHT): a randomised, double-blind, placebo-controlled, phase 2 study. Lancet Oncol. 2022;23(11):1430–40.
-
Fuchs CS, Tomasek J, Yong CJ, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383(9911):31–9.
-
Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15(11):1224–35.
-
Drigo JM, Castillo C, Wever W, et al. Endoscopic ultrasound practice survey in Latin America. Endosc Ultrasound. 2013;2(4):208–18.
-
Durán CE, Cañás M, Urtasun M, et al. Potential negative impact of reputed regulators’ decisions on the approval status of new cancer drugs in Latin American countries: a descriptive analysis. PLoS One. 2021;16(7):e0254585.
-
Werutsky G, Barrios CH, Cardona AF, et al. Perspectives on emerging technologies, personalised medicine, and clinical research for cancer control in Latin America and the Caribbean. Lancet Oncol. 2021;22(11):e488–500.
-
Acevedo AM, Gómez A, Becerra HA, et al. Distribution and trends of hematology and oncology research in Latin America: a decade of uncertainty. Cancer. 2014;120(8):1237–45.
-
Arai RJ, Guindalini RSC, Llera AS, et al. Personalizing precision oncology clinical trials in Latin America: an expert panel on challenges and opportunities. Oncologist. 2019;24(8):e709–19.
-
da Silva RE, Amato AA, Guilhem DB, et al. International clinical trials in Latin American and Caribbean countries: research and development to meet local health needs. Front Pharmacol. 2018;8:961.





