Invasive Cutaneous and Ocular Candidiasis in a Child with DOCK8 Deficiency: A Case Report
Abstract
Dedicator of Cytokinesis 8 (DOCK8) deficiency is a severe combined immunodeficiency that predisposes patients to recurrent and atypical infections. We report the case of an 8-year-old boy who presented with a rapidly progressive, fungating facial mass and keratomycosis, initially mimicking a neoplastic or mycobacterial process. An extensive diagnostic workup, including multiple biopsies, was necessary to identify Candida albicans as the causative pathogen. The severity and atypical nature of the infection prompted a comprehensive immunological evaluation, which revealed a biallelic pathogenic mutation in the DOCK8 gene. This case highlights the importance of meticulous microbiological and histopathological work-up and appropriate treatment of fungal infections with unusual clinical features.
Introduction
Dedicator of Cytokinesis 8 (DOCK8) deficiency is a rare, autosomal recessive combined immunodeficiency characterized by defective cellular and humoral immunity. Invasive candidiasis is a life-threatening infection that mainly affects immunocompromised hosts. Although candidemia is a common presentation, its occurrence as a rapidly progressing, fungating skin mass is very rare and often resembles malignancy or unusual infections, making diagnosis difficult. We report a challenging case of severe invasive cutaneous and ocular candidiasis in an 8-year-old boy, which ultimately led to the diagnosis of DOCK8 deficiency. To our knowledge, this is a rare case of invasive cutaneous candidiasis presenting as a fungating facial mass with concurrent keratomycosis as the initial manifestation of DOCK8 deficiency in a pediatric patient, highlighting a unique and severe diagnostic challenge. This case underscores the need to consider underlying primary immunodeficiencies in patients with atypical fungal infections and the pivotal role of aggressive diagnostics.
Case Presentation
An eight-year-old boy initially presented with a sudden onset and progressive course of a papillomatous lesion at the medial canthus of the right eye over two weeks. Three months later, the lesion rapidly progressed into a large, fungating facial mass, accompanied by multiple skin nodules, widespread rashes, intermittent fever, and visual impairment (Figure 1).
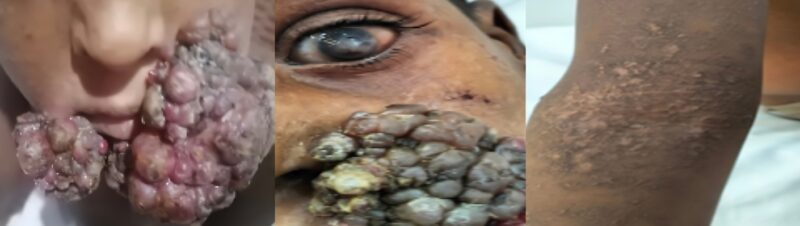
Figure 1: Fungating facial mass, skin rash and opacity in both eyes
During the clinical examination, the patient was fully conscious, with average weight and height for his age, and exhibited no abnormal facial features. He had a high-grade fever (39°C) and a large fungating mass involving the area behind the right ear, the medial canthus of the right eye, and both corners of the mouth. Multiple skin nodules (up to 1×2 cm in size) were observed on the neck and genital region, along with a generalized skin rash. Visual assessment showed impaired vision in both eyes, with perception of light and finger counting at 2 meters. Since infancy, the patient had experienced recurrent respiratory infections and chronic skin rashes, previously diagnosed as eczema. There was no family history of consanguinity, malignancy, blood transfusion, or prior hospitalizations.
Considering the patient’s history and clinical presentation, the initial differential diagnoses included both infectious and neoplastic causes. The infectious causes considered were atypical mycobacterial infection, bacillary angiomatosis, fungal infection, molluscum contagiosum, and tuberculous infection. The neoplastic causes included lymphoma, mycosis fungoides (a subtype of cutaneous T-cell lymphoma), and malignant soft tissue sarcoma.
A comprehensive diagnostic workup was requested, including complete blood count, liver and renal function tests, blood cultures with sensitivity, inflammatory markers, viral serology, PET/CT (Figure 2), excisional biopsy of the facial lesion, fundus examination, orbital ultrasound, corneal scraping, as well as immunological and genetic testing.
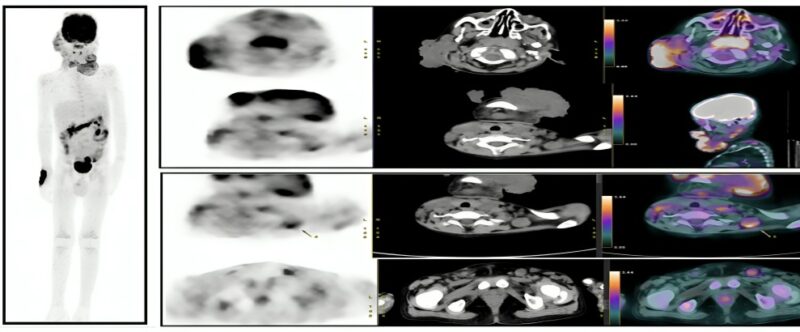
Figure 2: Multiple metabolically active facial soft tissue lesions with metabolically active lymph nodes
The primary result of the histological examination of the excised lesion suggested a chronic inflammatory process, raising suspicion for atypical mycobacterial infection (AMI) alongside deep fungal infection (Figure 3). Further special staining with PAS, GMS, ZN, and Gram’s stains is recommended to identify the nature of the causative organism.
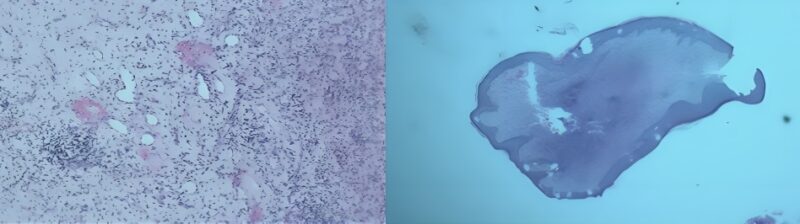
Figure 3: The primary result of the histological examination, AMI
Combined anti-tubercular therapy was initiated according to standard guidelines, and blood cultures and sensitivity tests for bacterial, fungal, and mycological evaluation were obtained. After seven days, the patient remained febrile, with enlargement of existing lesions and the appearance of new ones. Empirical antifungal therapy with fluconazole was initiated, in addition to symptomatic management, including antipyretics, antihistamines, and topical antifungal and antibacterial agents. Despite these measures, the facial mass continued to enlarge, accompanied by bleeding and a foul odor (Figure 4).
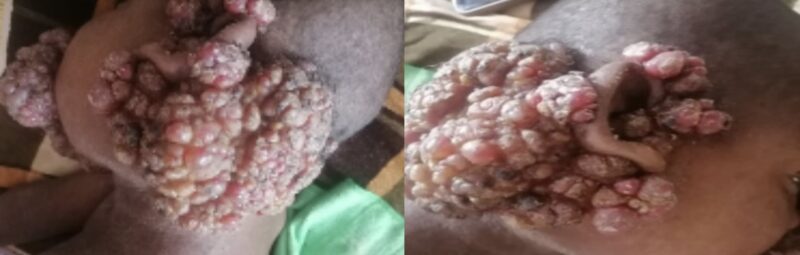
Figure 4: Progressive fungating facial masses
The patient’s condition progressively deteriorated, with persistent high-grade fever, decreased oral intake, generalized edema, and oliguria, prompting urgent hospital admission.Laboratory investigations revealed leukocytosis, microcytic hypochromic anemia with anisocytosis, eosinophilia, and monocytosis. C-reactive protein (CRP) was elevated, while liver and kidney function tests were within normal limits. Viral serology for HBV, HCV, and HIV was negative. Non-culture-based fungal biomarkers, such as β-D-glucan and PCR, were not performed, as these tests were unavailable at our institution and prohibitively expensive externally. Blood cultures were drawn before initiating empirical broad-spectrum antimicrobial therapy per institutional protocols, consisting of intravenous cefepime, vancomycin, and therapeutic voriconazole, along with supportive care.
Histopathological examination of the lesion biopsy confirmed Candida albicans, with susceptibility to voriconazole and itraconazole, and intermediate sensitivity to fluconazole, ketoconazole, and terbinafine. On the third day of hospitalization, the patient continued to have a high-grade fever; therefore, a low dose of corticosteroids was administered to address possible immune-mediated inflammation, resulting in marked clinical improvement. Fever and inflammatory markers normalized within seven days of initiating treatment.
A total of three blood cultures obtained during hospitalization were negative for both bacterial and fungal pathogens. After ten days of antimicrobial therapy, the regimen was de-escalated to oral voriconazole. Following clinical stabilization, the patient was discharged after two weeks of hospital admission on prophylaxis oral voriconazole with a plan for level-guided dose adjustment, along with trimethoprim-sulfamethoxazole, topical ocular therapy, dermatologic topical treatments, and oral antihistamines.
A comprehensive Candida workup was performed, including echocardiography, abdominal and pelvic ultrasound, and chest CT, all of which revealed no abnormal findings. Further evaluation of visual impairment, including fundus examination and bilateral orbital ultrasound, showed no significant abnormalities except for mild edema of the right optic nerve head. Corneal tissue obtained by corneal scraping was sent for histopathological analysis, and topical voriconazole was initiated along with prophylactic antibiotic eye drops and lubricants.
Regular outpatient follow-up demonstrated sustained clinical improvement, with all lesions showing a regressive course. The follow-up period remained uneventful for up to six months (Figure 5).
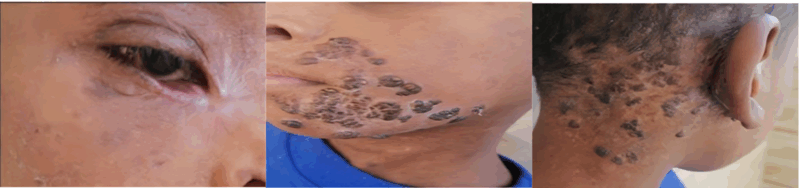
Figure 5: Regular follow and marked clinical improvement
During follow-up, the patient developed several recurrent infections, including upper respiratory tract infections, otitis media, urinary tract infection, and multiple skin abscesses. Routine blood investigations remained within normal limits. Culture and sensitivity testing identified Escherichia coli and Pseudomonas aeruginosa as the most common pathogens. Both infections responded well to targeted antibiotic therapy, with complete clinical recovery.
Immunological testing was performed, including complete blood count, dihydrorhodamine (DHR) assay, quantitative immunoglobulins (IgG, IgA, IgM), total complement activity, and lymphocyte phenotyping (CD3, CD4, CD45, CD8, CD19, CD20, and natural killer cells CD16/CD56). Results showed elevated DHR, increased IgG and IgA levels, and a relative decrease in CD4⁺ cells and the CD4/CD8 ratio, indicating underlying immunosuppression suggestive of a primary immunodeficiency syndrome. Whole exome sequencing confirmed DOCK8 deficiency.
The patient was referred to immunology and transplantation specialists for further evaluation and planning of hematopoietic stem cell transplantation from an allogeneic donor as a curative treatment after infection resolution, ophthalmology for keratoplasty or corneal transplantation, tissue viability and necrosis teams for continued coordination, and plastic surgery for potential reconstructive and cosmetic interventions involving the face and right lower eyelid.
Discussion
Fungi are eukaryotic organisms commonly found in decaying vegetation, soil, and air. Several species can cause severe infections in humans, especially in immunocompromised hosts. Among these, Candida species are among the most common causes of disseminated bloodstream infections, along with Staphylococcus aureus, coagulase-negative staphylococci, and Enterococcus species. The prevalence of candidiasis varies widely across different geographic areas, healthcare settings, and patient groups, often due to underlying risk factors. Invasive candidiasis (IC) usually originates from endogenous sources and is triggered by a failure in the host’s immune defenses1,2.
Diagnosis is frequently challenging due to the nonspecific nature of clinical signs and symptoms, which can range from fungemia to deep-seated candidiasis and, in severe cases, septic shock accompanied by multiorgan failure. A high index of suspicion must be maintained, as delays in diagnosing fungal infections may result in significant morbidity or even mortality2,3.
Cutaneous manifestations of disseminated candidiasis commonly present as asymptomatic erythematous to violaceous papules with pale, vesicular centers, which may progress to necrotic purpuric plaques or tense hemorrhagic bullae. Fungal keratitis (keratomycosis), a serious ocular infection often seen in debilitated patients, is typically slow progressing but can lead to severe visual impairment. Approximately 40% of cases are trauma-related, and around 10% are caused by yeasts—Candida albicans being the most frequent. Diagnosis is frequently delayed due to nonspecific and varied clinical features, particularly in the early stages. Although symptoms resemble those of other corneal infections, lesions may appear indolent in some cases, further complicating timely recognition and treatment4,5.
Blood culture remains the gold standard for diagnosing candidemia, with a sensitivity ranging from 21% to 71%2. The median time to positivity is approximately 2–3 days, but fungal growth may take up to 8 days. Positive histopathological findings from normally sterile sites can also support the diagnosis. Additionally, several non-culture-based antigen and antibody assays—such as the β-D-glucan assay and a real-time quantitative PCR assay—are available to aid in detecting invasive candidiasis7. Conventional microbiological techniques—like light microscopy and culture—can generally identify Candida species, but the presence of coexisting bacterial pathogens in clinical specimens may inhibit fungal growth. Therefore, combining multiple diagnostic approaches may improve both the sensitivity and timeliness of diagnosis2,3.
Diagnostic imaging plays a key role in invasive fungal disease (IFD), including early detection, assessment of dissemination, monitoring treatment response, and identifying relapse. CT and MRI are essential for evaluating disease extent and guiding surgical decisions. FDG PET/CT is emerging as a useful tool for detecting infection and monitoring therapeutic response. Although its cost may limit routine use, it can help localize clinically occult infections and evaluate treatment efficacy—particularly when patients show clinical improvement despite persistent lesions on conventional imaging. Thus, FDG PET/CT may serve as a valuable adjunct in managing invasive fungal infections5,6,7.
Broad-spectrum antifungal therapy should be initiated promptly when there is a high clinical suspicion of invasive fungal infection. The selection of the initial antifungal agent should take into account several factors, including the severity of the disease, local epidemiological patterns, prior antifungal exposure, underlying comorbidities such as neutropenia, and the presence of disseminated end-organ involvement. Currently available antifungal classes include polyenes, azoles, and echinocandins, each with distinct mechanisms of action and indications. International clinical guidelines emphasize that earlier initiation of antifungal therapy is associated with improved clinical outcomes. They recommend continuing treatment for at least 14 days after the first negative blood culture, provided that metastatic complications are absent and clinical resolution is achieved5,7.
In this case, the patient showed only intermediate sensitivity to fluconazole, while demonstrating full susceptibility to voriconazole, which corresponded clinically to a rapid and sustained improvement. This highlights the importance of antifungal susceptibility testing in guiding targeted therapy and improving patient outcomes. Fluconazole, although commonly used as a first-line antifungal agent, has limited activity against some Candida species and may show reduced efficacy in cases with intermediate sensitivity. Voriconazole, a second-generation triazole, exhibits broader antifungal coverage and greater potency against Candida albicans, particularly in resistant or refractory infections7.
Management of fungal keratitis typically involves topical and/or systemic antifungal medications, often combined with surgical intervention and prophylaxis against secondary bacterial infections. However, the range of available ocular antifungal agents is limited, and many lack sufficient corneal penetration, reducing their effectiveness. Newer therapeutic options, such as oral voriconazole and topical amphotericin B, have shown promise in selected cases. Despite treatment, complete healing may take weeks to months, and outcomes can be poor. Severe visual impairment occurs in approximately 26% to 63% of patients, with 15% to 20% requiring evisceration. Penetrating keratoplasty is necessary in about 31% to 38% of cases4,8,9.
Immunosuppression is a critical determinant in the pathogenesis of IC. Genetic variations in immune-related genes can either increase susceptibility or confer protection. During IC, the immune system recognizes pathogen-associated molecular patterns (PAMPs), triggering neutrophil recruitment and proinflammatory cytokine production. Activation of neutrophils, monocytes, macrophages, and dendritic cells leads to the production of protective cytokines and chemokines, enhanced recruitment and phagocytic activity of immune cells, generation of reactive oxygen species (ROS), and increased fungal killing capacity. These mechanisms also influence T-helper cell differentiation. In some patients, elevated interleukin-10 (IL-10) and reduced IL-12B levels have been associated with disease progression despite antifungal therapy2,3.
This case highlights the significance of underlying primary immunodeficiencies in the pathogenesis and severity of invasive fungal infections. Whole-exome sequencing identified a pathogenic mutation in the Dedicator of Cytokinesis 8 (DOCK8) gene, confirming a diagnosis of autosomal recessive DOCK8 deficiency. DOCK8 deficiency is a rare, progressive form of combined immunodeficiency characterized by both humoral and cellular immune dysfunction. It is typically associated with peripheral eosinophilia, elevated serum IgE levels, and recurrent cutaneous infections of bacterial, viral, and fungal origin. Patients are also at increased risk for severe systemic infections, immune dysregulation, and malignancies, contributing to high morbidity and mortality10.
A recent Indian study reviewed 17 patients with Dedicator of Cytokinesis 8 (DOCK8) deficiency over five years and characterized the spectrum of infections. Infections were frequent, recurrent, and involved multiple organ systems. Bacterial infections predominated (~70%), with Pseudomonas aeruginosa and Staphylococcus aureus (both MRSA and MSSA) being the most common pathogens, particularly associated with respiratory, cutaneous, and systemic infections. Other isolates included Escherichia coli, Enterobacter cloacae, Streptococcus pneumoniae, and Acinetobacter species, causing urinary, gastrointestinal, and invasive infections. Viral infections (~25–30%) were mainly mucocutaneous and respiratory, caused by HSV, RSV, Rhinovirus, H1N1, and CMV, reflecting impaired antiviral immunity. Fungal and parasitic infections (<10%) involved Candida species and Cryptosporidium, primarily affecting mucosal and gastrointestinal sites11.
Early recognition of Dedicator of Cytokinesis 8 (DOCK8) deficiency is crucial. Management of infectious complications includes the use of targeted antimicrobial therapies (antibacterial, antifungal, and antiviral agents) during acute episodes, as well as long-term prophylaxis. Adjunctive therapies such as intravenous immunoglobulin (IVIG) and interferon-α2b may further support immune function. Hematopoietic stem cell transplantation (HSCT) is the definitive, curative therapy that has fundamentally altered the disease’s trajectory. The long-term survival benefits are profound and well-documented, with modern approaches achieving survival rates exceeding 80%. Beyond survival, HSCT resolves the debilitating infections, allergies, and eczema that define the disease, allowing patients to lead healthy, productive lives10,12.
Conclusion
Atypical and invasive cutaneous candidiasis should be considered a potential harbinger of an underlying primary immunodeficiency early identification and genetic confirmation of such conditions are essential for implementing curative strategies, such as hematopoietic stem cell transplantation, and for minimizing long-term complications.
Conflict of Interest
The authors declare that there is no conflict of interest regarding the publication of this case report.
Consent statement
The authors declare that signed consent to publish was obtained from the legal guardian (mother) of the patient regarding the publication of data and findings related to this study.
License
© Author(s) 2025.
This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, and unrestricted adaptation and reuse, including for commercial purposes, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
To view a copy of this license, visit https://creativecommons.org/licenses/by/4.0/.
References
-
Lass-Flörl C, Kanj SS, Govender NP, Thompson GR 3rd, Ostrosky-Zeichner L, Govrins MA. Invasive candidiasis. Nat Rev Dis Primers. 2024 Mar 21;10(1):20.
-
Pappas PG, Lionakis MS, Arendrup MC, Ostrosky-Zeichner L, Kullberg BJ. Invasive candidiasis. Nat Rev Dis Primers. 2018 May 11;4:18026.
-
Gonzalez-Lara MF, Ostrosky-Zeichner L. Invasive Candidiasis. Semin Respir Crit Care Med. 2020 Feb;41(1):3-12.
-
Mukherjee B, Raichura ND, Alam MS. Fungal infections of the orbit. Indian J Ophthalmol. 2016 May;64(5):337-45
-
Mezri S, et al. Case report: invasive candidiasis of the head and neck in a five-month-old infant: a case study. F1000Res. 2024;13:1232.
-
Douglas AP, Thursky KA, Worth LJ, Drummond E, Hogg A, Hicks RJ, Slavin MA et al. FDG PET/CT imaging in detecting and guiding management of invasive fungal infections: a retrospective comparison to conventional CT imaging. Eur J Nucl Med Mol Imaging. 2019 Jan;46(suppl 1):166–173.
-
Pappas PG, Kauffman CA, Andes DR, et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2016 Feb 15;62(4):e1-50.
-
Alcaraz-Micheli L, Moon C. Fungal keratitis. American Academy of Ophthalmology EyeWiki. 2014, May
-
Awad R, Ghaith AA, Awad K, Mamdouh Saad M, Elmassry AA. Fungal Keratitis: Diagnosis, Management, and Recent Advances. Clin Ophthalmol. 2024 Jan 10;18:85-106.
-
Ollech A, et al. Treatment options for DOCK8 deficiency-related severe dermatitis. J Dermatol. 2021 May 27;48(9):1386–1393.
-
Gowri V, Chougule A, Gupta M, et al. Clinical, immunological and molecular findings of patients with DOCK-8 deficiency from India. Scand J Immunol. 2023 Jul;98(1):e13276.
-
Freeman AF, Gonzalez CE, Yates B et al. Hematopoietic cell transplantation for DOCK8 deficiency: results from a prospective clinical trial. J Allergy Clin Immunol. 2025 Jan;155(1):176-187.





