Autologous Hematopoietic Stem Cell Transplant in Pediatric Solid Tumors in India: A Single Center Experience of 15 Years
Abstract
Introduction: High-dose chemotherapy followed by autologous hematopoietic stem cell transplant (ASCT) remains a curative option for many pediatric solid tumors when standard-dose chemotherapy fails. The data on ASCT from low-and middle-income countries (LMICs), including India, remains sparse and limited to mostly neuroblastoma.
The primary objective of this study was to evaluates the outcomes of ASCT in children, adolescents, and young-adults (CAYA,0-18yrs) with solid tumors treated at a tertiary care center in India over 15 years and to assess the survival outcomes, including overall-survival (OS) and event-free-survival (EFS). Secondary objectives included evaluating engraftment-kinetics, treatment-related toxicities, and the impact of ASCT on tumor types.
Methodology: Data collection was done from hospital files, electronic databases and patient follow-up records for cases, who underwent ASCT for solid tumors between January 2008 and August 2024.
Results: Out of 84 patients, median age was 6y (2-24) with 70.7% males. The diagnoses included: neuroblastoma (NB,52%), relapsed/refractory germ-cell tumors (GCT,15.5%), relapsed/refractory ewing sarcoma (EWS,22.6%), retinoblastoma (RB) and soft-tissue sarcoma (STS) 4.7% each. While 41 (48.8%) patients underwent ASCT during relapse, rest 43 (51.2%) patients underwent upfront ASCT (NB). The conditioning regimens were: busulfan-melphalan or carboplatin-etoposide-melphalan for NB, melphalan or busulfan-melphalan for EWS, carboplatin-etoposide for GCT and thiotepa-carboplatin for STS/RB. Median CD34+ stem cell dose was 4.6 (1.8-20.1) million/kg, with a median cryopreservation duration of 13.5 (4-110) days. Toxicity details are given in Table-1.
Table 1: Clinical details of the autologous hematopoietic stemcell transplant in pediatric solid tumors
| Col 1 | Col 2 | |
|---|---|---|
| Median age= 6y (2-24) Sex: Male= 70.7% Indication and time of transplant First remission= 43 (51.2%) Relapse or refractory (post salvage)= 41 (48.8%) | Diagnosis n= 84 (%) Neuroblastoma= 44 (52.4%) Ewing sarcoma= 19 (22.6%) Germ cell tumor= 13 (15.5%) Retinoblastoma= 4 (4.7%) Soft tissue sarcoma= 4 (4.7%) | |
| Cell dose and engraftment details | ||
| Median CD34 dose in 106/kg= 4.6 (1.8-20.1) Median duration of cryopreservation= 13.5 days (4-110) Median hospitalization duration= 23 days (12-42) Median time to ANC engraftment= 11 days (9-25) Median number of PRBC transfusion needed= 1 (1-5) Median number of platelets (SDP) transfusion needed= 2 (1-15) Median duration of antibiotics days= 10 days (5-42) Median duration of follow up= 441 days (9-2000) | ||
| Toxicity (n=54) | ||
| Infectious complications Staphylococcus aureus= 4 Acinetobacter baumani= 3 Enterococcus faecium= 3 Escherichia coli= 1 Clostridium difficile= 1 Giardia lamblia= 1 Candida sp.=1 | Non-infectious complications (≥Grade 3) Mucositis= 22 Chemotherapy induced nausea vomiting= 12 Diarrhoea= 16 Acute kidney injury (any grade)= 3 Transplant related mortality (TRM)= 3 (3.5%) | |
Median time to neutrophil-engraftment was 11 days (9-25). With a median follow-up of 441days estimated 1-year overall-survival (OS) and event-free-survival (EFS) for the entire cohort were 77.0% and 58.4% respectively; better in GCT than NB or EWS (Figure-1). BuMel-140 had better outcomes than CEM-200 or Melphalan-200 [HR=0.5,95%CI:0.3-0.8].
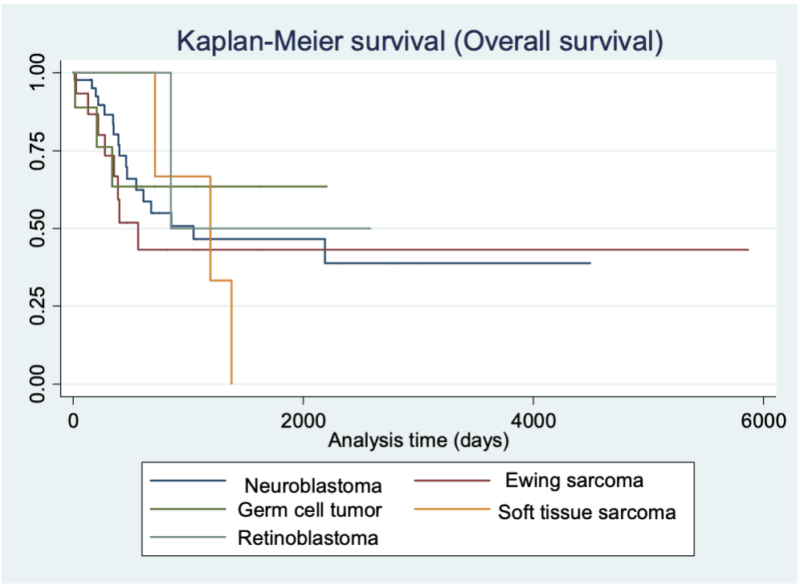
Figure 1: Overall survival of pediatric solid tumors after autologous stem cell transplant
The study highlights ASCT as a viable therapeutic option for pediatric solid tumors in LMICs, demonstrating favorable outcomes comparable to international standards. Challenges include optimizing supportive care to minimize toxicities and improving accessibility. The superior outcomes in GCT underline the potential of ASCT in achieving long-term remission in chemosensitive tumors.
Conclusion: ASCT can be safely performed in resource-limited settings, offering curative potential for pediatric patients with high-risk or relapsed solid tumors. Expanding access and refining protocols in LMICs could bridge the gap in global cancer care outcomes.