Survival Outcomes of Wilms’ Tumor in Pediatric Patients Treated at KFMC: A Retrospective Study of 15 years from 2007-2022
Abstract
Background: Wilms’ tumor is the most common malignant renal tumor in children, with high cure rates in high-income settings due to standardized treatment protocols. However, outcomes in different regions can vary based on treatment approaches and resource availability. This study aims to evaluate the overall survival and relapse-free survival of pediatric patients with Wilms’ tumor treated at King Fahad Medical City over a 15-year period and compare institutional outcomes with national and international outcomes.
Methods: A retrospective cohort study was conducted, including 94 pediatric patients diagnosed with Wilms’ tumor and treated at KFMC between July 2007 and June 2022. Clinical, pathological, and treatment-related data were collected from hospital electronic systems. Survival analysis was performed using the Kaplan-Meier method.
Results: The median age at diagnosis was 62.5 months, with a female predominance (60.6%). A significant proportion (46.8%) presented with pulmonary metastasis, and over 56% had stage III or IV disease. Delayed nephrectomy following neoadjuvant chemotherapy was the predominant surgical approach (79.8%). Anaplastic histology was found in 6.4%, and blastemal predominance in 29.7%. At final follow-up, 95.7% of patients were alive, and 88.3% remained relapse-free. The mean overall survival (OS) was 169.4 months, and the mean recurrence-free survival (RFS) was 145.4 months.
Conclusion: This study highlights that adherence to international treatment protocols can lead to excellent survival outcomes in pediatric patients with Wilms’ tumor, even in cases diagnosed at advanced stages. Continued emphasis on protocol-based care, early diagnosis, and integration of molecular prognostics will be crucial in sustaining and improving outcomes.
Introduction
Wilms’ tumor (WT), or nephroblastoma, is the most prevalent malignant renal neoplasm in the pediatric population, accounting for approximately 90% of all childhood renal tumors. In contrast, other renal malignancies, such as clear cell sarcoma and renal cell carcinoma, occur far less frequently in children1. Due to its status as the most common solid intra-abdominal tumor in pediatrics, WT remained a central focus of clinical research aimed at optimizing therapeutic strategies and long-term outcomes.
Clinically, Wilms’ tumor often presents with an asymptomatic abdominal mass and, less commonly, with systemic manifestations such as fever, hypertension, or hematuria—the latter being a relatively rare initial symptom2. Histologically, WT arises from metanephric blastemal cells that fail to differentiate properly, leading to the development of classic triphasic tumors comprising epithelial, stromal, and blastemal components. Molecular studies have identified elevated levels of TERT RNA and overexpression of n-Myc as markers associated with increased risk of disease relapse, demonstrating the molecular complexity and prognostic variability of WT2.
Diagnostic evaluation typically begins with abdominal ultrasonography complemented by Doppler imaging to assess vascular involvement, followed by cross-sectional imaging for staging purposes3. Histopathologically, Wilms’ tumor is broadly classified into favorable histology (FH) and anaplastic histology (AH), the latter characterized by enlarged hyperchromatic nuclei, abnormal mitotic figures, and nuclear atypia, which may be focal or diffuse in distribution. The presence of diffuse anaplasia is associated with poor response to therapy and significantly reduced survival rates. The treatment approaches are influenced by tumor histology and stage, with favorable histologic subtypes generally associated with excellent outcomes, whereas anaplastic variants show significantly poorer prognoses, with 5-year survival rates reported around 50%4.
Two major international groups—the National Wilms’ Tumor Study Group/Children’s Oncology Group (NWTSG/COG) in North America and the International Society of Pediatric Oncology (SIOP) in Europe—have established evidence-based, standardized treatment protocols for Wilms’ tumor. A key distinction between these protocols is the timing of surgical intervention. The COG approach typically recommends immediate nephrectomy for most cases of unilateral disease, enabling accurate histological assessment and staging at the outset. In contrast, the SIOP protocol recommends administration of preoperative (neoadjuvant) chemotherapy in children older than six months, with the intent of reducing tumor size, minimizing the risk of intraoperative tumor rupture, and facilitating surgical resection through potential tumor downstaging5. Both groups recommend risk-adapted multimodal therapy, including surgery, chemotherapy, and radiotherapy, tailored to individual clinical and pathological risk factors. The primary therapeutic goal is to maintain high overall survival (OS) rates—approaching 90% in high-resource settings—while minimizing treatment-related toxicity1. Despite these successes, disease relapse remains a concern, with recurrence rates reported in approximately 15% of patients, as highlighted in recent studies6.
In Europe, the SIOP approach—emphasizing preoperative chemotherapy—has demonstrated efficacy in reducing tumor rupture and improving operability7. Although radiotherapy was initially a standard component of preoperative management, studies have shown that two-agent chemotherapy regimens can achieve similar outcomes, making chemotherapy the preferred modality. SIOP’s risk stratification further delineates tumors into low-, intermediate-, and high-risk categories, guiding treatment intensity accordingly8.
At King Fahad Medical City (KFMC), the institutional treatment protocol for Wilms’ tumor is aligned with the guidelines established by the Children’s Oncology Group (COG), incorporating a multimodal therapeutic approach that includes surgical resection, systemic chemotherapy, and radiotherapy. Chemotherapy protocol includes EE4A, DD4A, VAD/NSS, DD4A/Regimen M, UH1/UH2, Regimen I, and Umbrella protocol. Despite the advances in therapeutic regimens, long-term complications, particularly related to renal function, remain a challenge, given the cumulative effects of nephrectomy, chemotherapy, and abdominal irradiation.
This retrospective study aims to analyze the clinicopathological characteristics and treatment outcomes of pediatric patients diagnosed with Wilms’ tumor over 15 years at KFMC. By comparing our institutional data with regional and international benchmarks, we seek to evaluate the efficacy of current management strategies and identify areas for further optimization in the care of Saudi pediatric patients with WT.
Aims and objectives:
The primary objective of this study is to evaluate the survival outcomes of pediatric patients diagnosed with Wilms’ tumor who received treatment at King Fahad Medical City (KFMC) between 2007 and 2022. Specifically, the study aims to assess both overall survival (OS) and relapse-free survival (RFS) rates in this patient population and compare survival results from KFMC with those reported in national and international Wilms’ tumor studies to evaluate the efficacy of current management protocols at the institutional level.
Materials and Methods
Study Design and Setting
This was a retrospective cohort study conducted at King Fahad Medical City (KFMC), Riyadh, Saudi Arabia. The study included pediatric patients diagnosed with Wilms’ tumor (nephroblastoma) who were treated at KFMC over 15 years from July 2007 to June 2022.
Patient Selection
All pediatric patients diagnosed with Wilms’ tumor (nephroblastoma) and treated at KFMC during the specified period were eligible for inclusion. A total of 94 patients met the inclusion criteria which include those confirmed to have nephroblastoma and less than 14 years. Patients were diagnosed based on clinicopathological features, supported by computed tomography (CT) imaging and histopathological confirmation following biopsy or surgical resection.
Ethical Approval
Approval for this study was obtained from the Institutional Review Board (IRB) at KFMC before data collection. All patient data were handled with strict confidentiality and in compliance with institutional and ethical guidelines.
Data Collection
Following approval by the Institutional Review Board (IRB), data were extracted from the hospital’s electronic medical systems, including CORTEX, CENTRICITY, and HIM EPIC. Extracted data included demographic information, clinical presentation, tumor location and staging, histological subtype, presence of pulmonary metastasis, treatment procedures (surgery, chemotherapy, radiotherapy), and outcomes. Each patient’s data was entered into a structured Excel database for analysis. The data collection focused on survival status at the last follow-up, occurrence of relapse, duration of survival, and relapse-free intervals.
Treatment Protocols
Treatment regimens were primarily based on the Children’s Oncology Group (COG) and International Society of Pediatric Oncology (SIOP) protocols. Depending on disease stage and patient-specific factors, patients received either upfront or delayed nephrectomy. Chemotherapy and radiotherapy were administered based on stage, histology, and risk category, with radiotherapy directed at abdominal or pulmonary sites.
Statistical Analysis
Descriptive statistics were used to summarize patient demographics and clinical characteristics. Continuous variables were expressed as mean ± standard deviation or median with interquartile ranges, as appropriate. Categorical variables were expressed as frequencies and percentages. Survival outcomes were analyzed using the Kaplan-Meier method to estimate overall survival and relapse-free survival. All statistical analyses were performed using SPSS for Windows, with a significance threshold set at p<0.05.
Results
Patient Demographics
Over 15 years, a total of 94 pediatric patients diagnosed with Wilms’ tumor were treated at King Fahad Medical City. The patients’ ages at diagnosis ranged from 4 to 177 months, with a mean age of 74.4 ± 47.2 months. The median age was 62.5 months, indicating that most diagnoses occurred during early to middle childhood. At the time of the final follow-up, the patients’ ages ranged from 32 to 243 months, with a mean of 114.5 ± 53.9 months and a median of 104.5 months (Table 1).
The cohort showed a female predominance, with 60.6% of the patients being female and 39.4% male. Regarding tumor site, 50% of tumors were located in the left kidney, 40.4% in the right kidney, and 9.6% of patients had bilateral disease (Table 1).
Disease Presentation and Metastasis
At disease presentation, 46.8% of the patients had evidence of pulmonary metastasis, while 53.2% had no metastatic spread. This indicates that nearly half of the patients were diagnosed at an advanced stage of the disease. Tumor staging further supports this, with stage III and IV disease being the most commonly observed, accounting for 30.9% and 25.5% of cases, respectively. Stage II disease was seen in 24.5% of patients, while stage I and bilateral stage V tumors were each observed in 9.6% of the cohort (Table 1).
Histopathological Findings
Histologically, six patients (6.4%) had anaplastic Wilms’ tumor, while blastemal predominance was observed in 28 patients (29.7%). These histological patterns reflect a diverse tumor biology within the cohort.
Treatment Procedures
Most patients (79.8%) received delayed surgery after neoadjuvant chemotherapy, adhering to SIOP-like treatment protocols. Only 20.2% received upfront surgical resection. Radiotherapy was administered in 54.3% of patients. Among these, 26.6% received pulmonary radiation, 29.1% received flank radiotherapy, and 17.9% received whole-abdominal radiotherapy (Table 1).
Table 1. Descriptive statistics of patients with Wilms' Tumor
Clinical Outcomes and Survival
At the time of last follow-up, 95.7% of patients were alive, while only 4.3% (n=4) had succumbed to the disease. Relapse was recorded in 11.7% of patients, whereas 88.3% maintained remission (Table 1). These results indicate excellent disease control and long-term survival among the majority of patients.
Survival Analysis
The mean overall survival time was 169.4 months. The corresponding overall survival rate was 95.7%, reflecting the success of the therapeutic approach in this population (Figure 1). Similarly, the mean relapse-free survival was 145.4 months, with a relapse-free survival rate of 85.9% (Figure 2).
The Kaplan-Meier survival curves for both overall and relapse-free survival showed early stabilization, particularly after the 100-month mark, indicating a durable therapeutic effect and low risk of late recurrence. These curves further emphasized the reliability of the multimodal treatment strategy employed at KFMC.
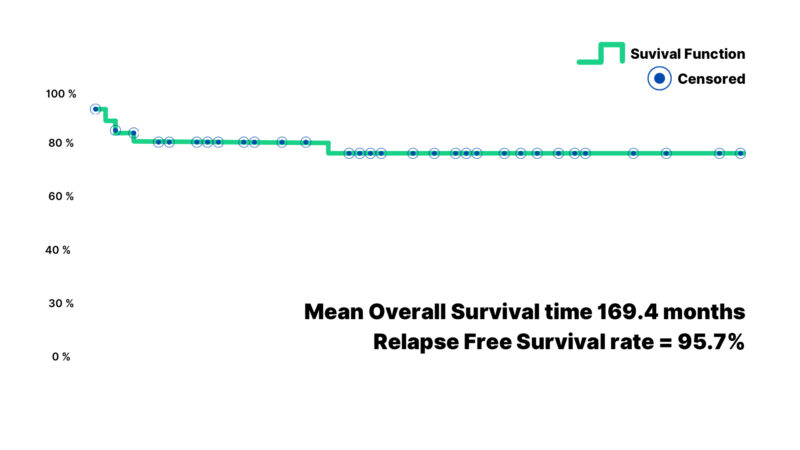
Figure 1: The graphical explanation of the overall survival time (months) of the Wilms’ tumor subjects demonstrating a mean survival time and the survival rate.
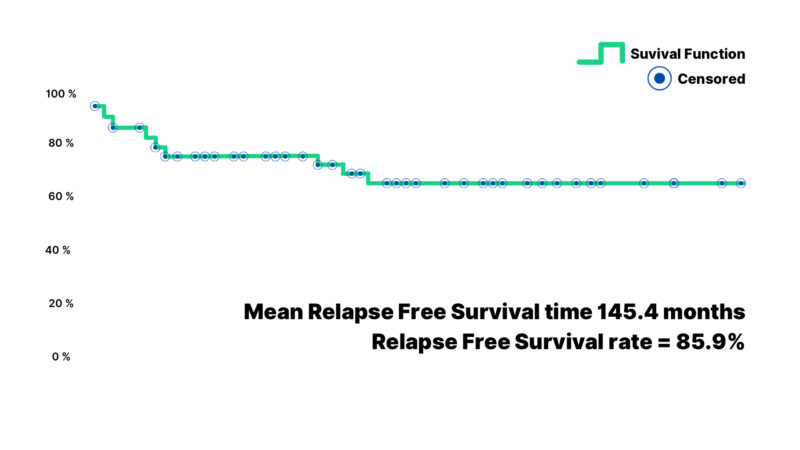
Figure 2: Graphical presentation of relapse-free survival time on the last follow-up (months) for Wilms’ tumor subjects depicting both the mean time of relapse-free survival and relapse-free survival rate.
Discussion
This study provides a comprehensive evaluation of survival outcomes among pediatric patients with Wilms’ tumor treated at King Fahad Medical City (KFMC) over 15 years. The results show promising overall survival (95.1%) and relapse-free survival (85.9%) rates, which are comparable to those reported in high-income countries implementing standardized protocols such as those developed by the Children’s Oncology Group (COG) and the International Society of Pediatric Oncology (SIOP). A comprehensive study conducted between 1975 and 2002 in the USA indicated that the 5-year survival rate for Wilms’ tumor in children younger than 12 years remained stable at approximately 90%.
Similarly, the survival rates observed in our study are comparable to those reported in a 2021 study by Alissa Groenendijk, Filippo Spreafico, and colleagues, which demonstrated an overall survival rate of approximately 90% 8, 9, 10. The low mortality rate (4.3%) and limited relapse incidence (11.7%) support the effectiveness of multimodal therapy combining chemotherapy, radiotherapy, and surgery. Our study demonstrated a lower relapse rate compared to international studies, which have reported relapse rates of approximately 15%, as noted by Abdel-Monem MM and El-Khawaga11 and Fichera et al.12. This reduced relapse rate in our cohort may be attributed to early tumor detection and the immediate implementation of appropriate treatment protocols.
Our cohort exhibited a slight female predominance (60.6%) and a peak age at diagnosis in early childhood (4-177 months, median 62.5 months), which aligns with the findings reported by Gooskens et al. The study highlighted that the most common age for the presentation of nephroblastoma is between 2 and 4 years, and less than 1% of all cases were diagnosed in individuals aged 18 years or older 4.
The presence of blastemal predominance in 29.7% of tumors is clinically significant, as this subtype is associated with inferior chemotherapy response and higher relapse rates if detected post-neoadjuvant treatment. However, relapse was not markedly elevated in this subset, potentially indicating effective risk-adapted optimization of therapy. Anaplasia, typically associated with worse outcomes, constituted a minority (6.4%) yet did not significantly diminish OS due to intensive multimodal therapy measures. A noteworthy observation was the higher-than-expected prevalence of bilateral disease (9.6%) in the cohort compared to the study conducted by Charlton et al. (2017), who reported it in 5-8% of patients13.
In this study, the majority of patients (79.8%) received delayed nephrectomy following neoadjuvant chemotherapy. This aligns with the SIOP protocol, which aims to reduce the risk of tumor rupture and enhance surgical outcomes. The favorable survival rates in our cohort further support the effectiveness of this strategy.
Radiotherapy was administered to more than half of the patients, with site-specific targeting based on metastatic spread and histological features. Pulmonary radiation was applied in 26.6% of cases, which is consistent with best practices for patients with pulmonary metastasis. Whole abdominal and flank radiation was used selectively, balancing the need for local control with the long-term risk of radiation-induced morbidity. The favorable relapse-free survival outcomes reinforce the value of such individualized, risk-adapted treatment planning.
Our study had certain limitations, notably the unavailability of cytogenetic data related to nephroblastoma at our institution and its retrospective design. Furthermore, we could not generate detailed epidemiological insights about the specific characteristics of Saudi pediatric patients with Wilms’ tumor due to the small cohort size. The retrospective nature of the study limited the granularity of some data, including molecular markers such as loss of heterozygosity at 1p/16q and gain of 1q, which have recently emerged as essential components of risk stratification.
Incorporating these biomarkers into future protocols could enhance prognostic accuracy. The relatively high proportion of patients presenting with advanced or metastatic disease suggests a need for improved awareness among primary care providers and earlier diagnostic interventions. Public health initiatives may further reduce delays in diagnosis and improve outcomes. Moreover, establishing national cancer registries and collaborative research frameworks can facilitate a better understanding of regional disease patterns and optimize treatment strategies.
Conclusion
This 15-year retrospective study at King Fahad Medical City demonstrates that pediatric patients with Wilms’ tumor can achieve excellent survival outcomes when treated according to international risk-stratified protocols. Despite a high incidence of advanced-stage and metastatic disease, the cohort experienced a 95.1% overall survival rate and an 85.9% relapse-free survival rate. These results demonstrate the effectiveness of a multimodal treatment approach that combines surgery, chemotherapy, and radiotherapy. Future efforts should focus on early diagnosis, incorporation of molecular risk factors, and assessment of long-term treatment-related effects to optimize both survival and quality of life.
Competing Interests: The authors declare no conflicts of interest related to this study. The study was conducted in accordance with the ethical standards outlined by the institutional review boards of the participating centers. There are no financial or personal relationships with other people or organizations that could inappropriately influence (bias) the study.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
License
© Author(s) 2025.
This is an open-access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, and unrestricted adaptation and reuse, including for commercial purposes, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
To view a copy of this license, visit https://creativecommons.org/licenses/by/4.0/.
References
-
Groenendijk A, Spreafico F, de Krijger RR, et al. Prognostic factors for Wilms tumor recurrence: a review of the literature. Cancers (Basel). 2021 Jun 23;13(13):3142.
-
Zhu S, Hu J, Cui Y, et al. Knockdown of SENP1 inhibits HIF-1α SUMOylation and suppresses oncogenic CCNE1 in Wilms tumor. Mol Ther Oncolytics. 2021 Jul 21;23:355-66.
-
Dome JS, Mullen EA, Dix DB, et al. Impact of the first generation of Children’s Oncology Group clinical trials on clinical practice for Wilms tumor. J Natl Compr Canc Netw. 2021 Aug 1;19(8):978-85.
-
Gooskens SL, Segers H, Pritchard-Jones K, et al. The clinical relevance of age at presentation in nephroblastoma. In: van den Heuvel-Eibrink MM, editor. Wilms tumor [Internet]. Brisbane (AU): Codon Publications; 2016 Mar. Chapter 2.
-
Bhutani N, Kajal P, Sharma U, et al. Many faces of Wilms tumor: recent advances and future directions. Ann Med Surg (Lond). 2021 Mar 7;64:102202.
-
Jablonowski CM, Gil HJ, Pinto EM, et al. TERT expression in Wilms tumor is regulated by promoter mutation or hypermethylation, WT1, and N-MYC. Cancers (Basel). 2022 Mar 25;14(7):1655.
-
Meier CM, Furtwängler R, Mergen M, et al. Impact of time to surgery on outcome in Wilms tumor treated with preoperative chemotherapy. Cancers (Basel). 2023 Feb 27;15(5):1494.
-
Spreafico F, Fernandez CV, Brok J, et al. Wilms tumour. Nat Rev Dis Primers. 2021 Oct 14;7(1):75.
-
Axt J, Abdallah F, Axt M, et al. Wilms tumor survival in Kenya. J Pediatr Surg. 2013 Jun;48(6):1254-62.
-
Tan X, Wang J, Tang J, et al. A nomogram for predicting cancer-specific survival in children with Wilms tumor: a study based on SEER database and external validation in China. Front Public Health. 2022 Apr 7;10:829840.
-
Abdel-Monem MM, El-Khawaga OY, Awadalla AA, et al. Gene expression analysis and the risk of relapse in favorable histology Wilms’ tumor. Arab J Urol. 2022 Sep 26;21(1):45-51.
-
Fichera G, Bisogno G, Affinita MC, et al. Lung relapse pattern in children with metastatic Wilms tumor. Pediatr Blood Cancer. 2024 Apr;71(4):e30856.
-
Charlton J, Irtan S, Bergeron C, et al. Bilateral Wilms tumour: a review of clinical and molecular features. Expert Rev Mol Med. 2017 Jul 18;19:e8.