Ecthyma Gangrenosum in a Pediatric Leukemia Patient
Abstract
Introduction: A 12-year-old female presented with a progressive skin lesion diagnosed as ecthyma gangrenosum. The growth of Pseudomonas aeruginosa was confirmed based on lesion progression, positive blood cultures, the patient’s immunocompromised status, and tissue biopsy results. The lesion was managed and treated with intravenous antibiotics and antifungals, along with conservative daily dressings.
Case Presentation: A 12-year-old girl with relapsed leukemia was admitted to the ICU with altered consciousness, hypotension, and hemorrhagic skin lesions. She had experienced a week of fever, bone pain, along with respiratory and gastrointestinal symptoms. Laboratory tests indicated elevated inflammatory markers and Pseudomonas aeruginosa bacteremia. Empirical antibiotics were initiated. After stabilization, she was transferred to the ward but developed new necrotic skin lesions. Voriconazole levels were normal. Ciprofloxacin and later gentamicin were added after sensitivity results confirmed a high-level AmpC-producing Pseudomonas. After two weeks, the skin lesions improved, and laboratory tests normalized. She was discharged after 25 days on oral cotrimoxazole and ciprofloxacin with follow-up.
Discussion: Ecthyma gangrenosum (EG) is a rare cutaneous infection that primarily occurs in immunosuppressed patients, leading to considerable morbidity and mortality. It is caused by bacteria, fungi, and viral infections. P. aeruginosa quickly spreads, resulting in single or multiple necrotic skin lesions that may involve one or more areas of the body. Diagnosis is based on skin biopsy and blood cultures. A multidisciplinary team should be involved to improve outcomes. Combined antimicrobial treatment and surgical debridement should be considered standard care; however, medical therapy alone may be sufficient for some patients. The clinical response depends on factors such as underlying diseases, severity, type of infection, adequate source control, and antibiotic response. Local antiseptic agents, such as citric acid, are recommended for their antibacterial properties and role in enhancing wound healing.
Conclusion: EG is an infectious complication seen in pediatric patients undergoing treatment for hematological malignancies. Prompt recognition and timely, adequate treatment are crucial for prognosis.
Introduction
Ecthyma gangrenosum (EG) is a rare but severe cutaneous infection that most often occurs in immunocompromised individuals. It is characterized by rapidly evolving necrotic ulcers surrounded by erythematous or violaceous margins. While Pseudomonas aeruginosa is the most frequently identified pathogen, other organisms, such as Aeromonas hydrophila, Escherichia coli, Klebsiella species, and Candida species, have also been implicated in its etiology. Among these, P. aeruginosa is most commonly associated with EG and carries the highest mortality, particularly in neutropenic patients. Reported mortality rates vary widely, with estimates ranging from 0 to 28% in localized infections and 38-70 % in cases with bacteremia. Early diagnosis and prompt initiation of appropriate antimicrobial therapy are critical to improving clinical outcomes. Here, we present a case of EG in a patient with relapsed acute lymphoblastic leukemia, emphasizing the importance of a multidisciplinary and timely therapeutic approach to optimize prognosis in this vulnerable population.
Case Presentation
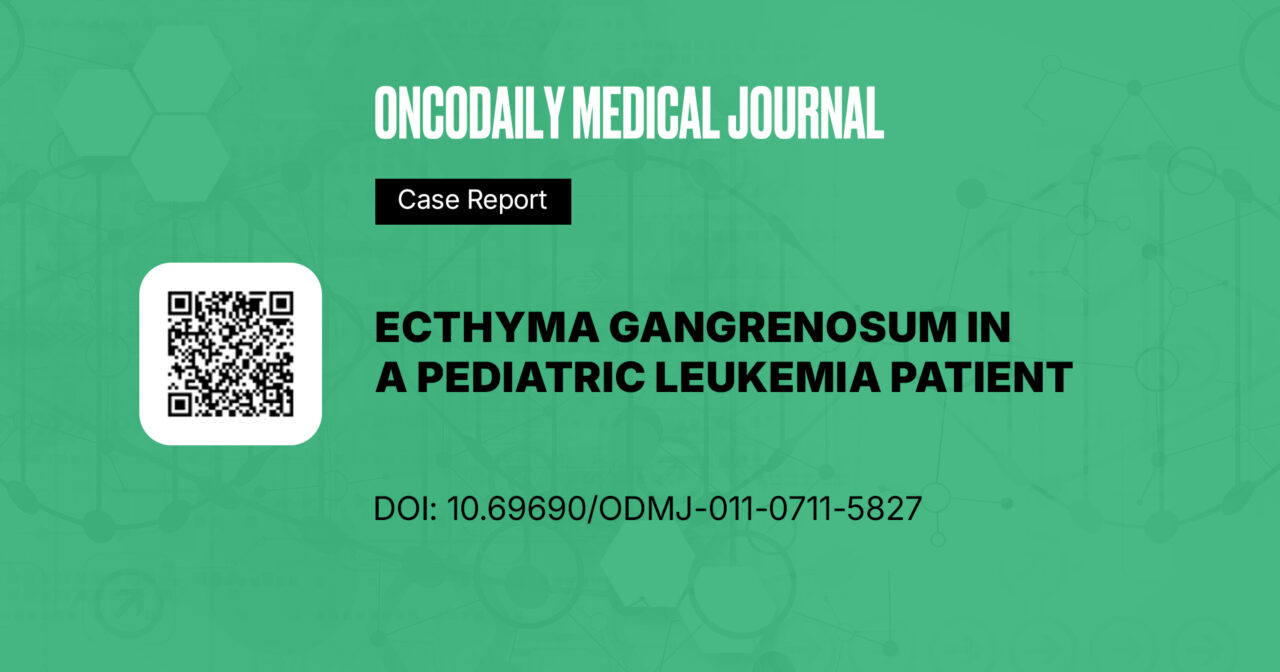
A 12-year-old girl receiving maintenance therapy for relapsed acute lymphoblastic leukemia (St. Jude ALL-R16 protocol) was admitted to the intensive care unit with altered consciousness, hypotension, and hemorrhagic skin lesions involving both upper and lower extremities (Figure 1). Her medical history was notable for a week of severe bone pain, accompanied by fever, ear discharge, rhinorrhea, cough, epistaxis, and gastrointestinal symptoms.
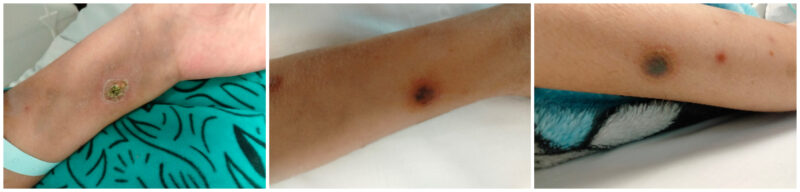
Figure 1: Initial presentation, skin lesions in both upper and lower extremities
On admission, comprehensive investigations revealed neutropenia (absolute neutrophil count <300/µL), leukopenia (WBC 0.6 ×10⁹/L), thrombocytopenia, elevated C-reactive protein (CRP 240 mg/L), and abnormal liver and kidney function tests. Cultures were promptly obtained before initiating antimicrobial therapy (blood, wound, and ear discharge). Tissue culture from the skin lesions yielded Staphylococcus aureus, mostly contaminated, while blood and ear discharge cultures were positive for Pseudomonas aeruginosa. The isolated Pseudomonas aeruginosa strain was sensitive to most anti-pseudomonal agents, including amikacin, cefepime, ceftazidime, ciprofloxacin, colistin, gentamicin, levofloxacin, meropenem, piperacillin/tazobactam, and imipenem.
Resistance was observed against amoxicillin/clavulanate, ceftriaxone, cefoxitin, trimethoprim/sulfamethoxazole, and tigecycline. Other potential infection sources, including urine culture and chest imaging, were evaluated and found to be negative—no central venous catheter (ruling out catheter-related bloodstream infection as a source).
Given the patient’s critical condition, characterized by hemodynamic instability, persistent fever, neutropenia, and the risk of polymicrobial and fungal infections, supportive treatment and empirical broad-spectrum antimicrobial therapy were initiated per institutional protocols and local antibiogram data. The regimen included high-dose meropenem (40 mg/kg/dose IV every 8 hours), amikacin (15 mg/kg/day IV or IM, once daily), vancomycin (15 mg/kg/dose IV every 6 hours), and liposomal amphotericin B (3– 5 mg/kg/day IV), along with supportive care.
After 48 hours in the intensive care unit, the patient’s general condition improved, and she was transferred to the ward for continued supportive treatment with ongoing clinical and laboratory monitoring. Ten days later, blood counts had recovered, and inflammatory markers had improved. A total of three blood cultures were obtained every three days during the initial febrile episode; they became negative after the second culture. As a result, empirical antimicrobial therapy was de-escalated to regular doses of meropenem (20 mg/kg/dose IV every 8 hours) and voriconazole (8 mg/kg orally every 12 hours).
However, a few days later, a new, progressive skin lesion with hemorrhagic necrosis appeared despite maintaining therapeutic voriconazole levels (Figure 2). The lesion evolved from an erythematous papule to a hemorrhagic bulla and then to a necrotic ulcer with black eschar over several days. This raised concerns about an ongoing or resistant infection. Intravenous ciprofloxacin was initiated based on previous P. aeruginosa sensitivity patterns. A surgical consultation was requested, and a tissue biopsy confirmed high-level AmpC-producing Pseudomonas aeruginosa. As a result, ciprofloxacin was replaced with gentamicin, which was administered alongside meropenem and voriconazole. Topical wound care with citric acid was also started.
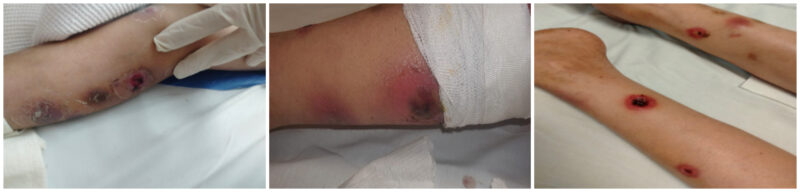
Figure 2: Progressive skin lesions with hemorrhagic necrosis
Over the following two weeks, the skin lesions demonstrated marked improvement (Figure 3). The total duration of antimicrobial therapy was 25 days. The patient was discharged on oral ciprofloxacin and prophylactic co-trimoxazole, with instructions for close follow-up at the outpatient clinic and adherence to good hygiene practices.
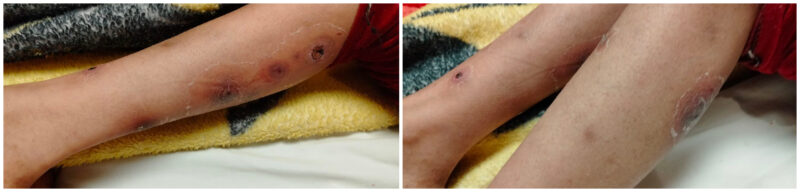
Figure 3: Marked improvement, after a total duration of 25 days of antimicrobial treatment
During outpatient follow-up, as reported by the primary consultant, the wound demonstrated progressive healing by secondary intention with complete epithelialization. The patient subsequently resumed and completed her chemotherapy protocol without further infectious complications.
Discussion
Infectious complications remain a leading cause of morbidity and mortality in children receiving cancer therapy, accounting for up to two-thirds of treatment-related deaths during intensive chemotherapy. In immunocompromised patients, infections may arise from common community pathogens, opportunistic organisms, or reactivation of latent infections1.
Gram-negative infections remain a major challenge due to increasing antimicrobial resistance. Among these, Pseudomonas aeruginosa is a leading cause of healthcare associated infections in critically ill and immunocompromised patients, often linked to high morbidity, mortality, and prolonged hospitalization. Pseudomonas aeruginosa is clinically significant because of its intrinsic resistance to many antibiotics, related to low outer membrane permeability and active efflux pumps. Its ability to form biofilms enhances persistence and reduces susceptibility to antimicrobial agents, making infections difficult to eradicate2,3.
Pseudomonas aeruginosa can cause a wide spectrum of skin and soft tissue infections, from mild to life-threatening forms. Among these, ecthyma gangrenosum (EG) is an aggressive necrotizing infection that mainly affects immunocompromised patients with hematologic malignancies, although it can rarely occur in healthy individuals. Several pathogens have been reported, but P. aeruginosa remains the most common causative agent4,5,6.
The development of EG is strongly associated with immunosuppression, particularly prolonged neutropenia, which allows bacterial translocation and promotes the emergence of resistant organisms. Additional risk factors include immunosuppressive therapy, mucositis, epithelial barrier disruption, microbiome dysbiosis, and long-term central venous catheter use. These factors collectively impair host defenses and increase susceptibility to opportunistic infections, often leading to delayed diagnosis due to atypical presentations5,7.
EG represents a form of septic vasculitis caused by direct microbial invasion of vascular walls, resulting in ischemic necrosis of the skin. P. aeruginosa produces virulence factors such as exotoxin A and elastase that contribute to endothelial damage and tissue necrosis. Lesions progress rapidly from erythematous macules to necrotic ulcers with black eschar. Histopathology typically shows vascular invasion, thrombosis, and necrosis with minimal inflammation in neutropenic patients5,8,9.
EG develops in about 1–30% of Pseudomonas aeruginosa sepsis cases. It usually starts as one or more painless erythematous lesions with papules or bullae that rapidly evolve into necrotic ulcers. The perineal region is most often affected (≈50%), followed by the extremities (≈30%). Uncommon sites include CVC exit points, bone marrow aspiration sites, and the periumbilical or scapular regions. As lesions may occur in hidden areas, thorough skin examination in high-risk patients is essential for early detection and management2,10.
In immunocompromised patients with Pseudomonas aeruginosa–related EG, rapid identification of the causative organism is critical. Diagnosis is based on skin biopsy and blood cultures. Management requires a multidisciplinary approach, typically involving three stages: initial empiric therapy, targeted antibiotics guided by culture results, and surgical intervention when indicated. Early initiation of appropriate antimicrobial therapy is vital to limit tissue damage. While neutropenia often limits surgical options, debridement remains important in extensive or progressive lesions2,10.
Antipseudomonal therapy commonly includes newer-generation cephalosporins such as ceftazidime and cefepime. In resistant P. aeruginosa infections, advanced agents like ceftolozane/tazobactam and ceftazidime/avibactam provide effective alternatives through β-lactamase inhibition. The usual duration of antibiotic therapy ranges from 10 to 14 days, adjusted according to clinical response and infection control2,10.
Tissue necrosis is a key feature of EG and often leads to delayed healing, scarring, and long-term cosmetic effects. Adjunctive measures such as negative-pressure wound therapy, hyperbaric oxygen, or dermal substitutes may promote granulation and improve wound recovery2,10,11. Topical antiseptics may help reduce microbial load in EG wounds, but their cytotoxicity limits routine use. Among available options, dilute acetic acid has shown effectiveness against P. aeruginosa and can aid in local infection control when used cautiously3,11.
The clinical outcome depends on several factors, including the patient’s comorbidities, infection severity, adequacy of source control, and appropriateness of antimicrobial therapy in terms of coverage, dosing, and duration11,12.
In this case, the patient achieved full recovery despite several high-risk features, including relapsed ALL, severe neutropenia, and hemodynamic instability. Early recognition, rapid initiation of targeted antipseudomonal therapy, and intensive supportive care were crucial for a favorable outcome. This case is notable for complete wound healing with medical therapy alone, without extensive surgical intervention. It highlights the potential for full recovery even in profoundly immunocompromised children. Early diagnosis and prompt multidisciplinary management remain essential to improve prognosis in such rare and severe infections.
Conclusion
Ecthyma gangrenosum is an infectious complication predominantly caused by Pseudomonas aeruginosa, occurring more frequently in immunocompromised or critically ill patients. Early recognition and prompt initiation of appropriate antimicrobial therapy are critical for improving patient outcomes. Combined antimicrobial and surgical interventions are considered the standard of care; however, in selected patients, medical therapy alone may be sufficient.
Conflict of Interest
The authors declare that there is no conflict of interest regarding the publication of this case report.
Ethical Considerations
Written informed consent for publication of this case and the accompanying clinical images was obtained from the patient’s parents. The case details were anonymized to ensure confidentiality. Approval for publication was obtained from the institutional Research and Publication Committee. The authors declare that this report does not conflict with any institutional or professional responsibilities.
License
This article is published under the terms of the Creative Commons Attribution 4.0 International License (CC BY 4.0).
© Walaa Ahmed Abdo Yahya, 2025. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
-
Acebo JJ, Bhattacharyya P, Escobedo-Melendez G, et al. Infections in immunosuppressed pediatric patients. In: Lakhoo K, Abdelhafeez AH, Abib S, editors. Pediatric Surgical Oncology. Cham: Springer; 2023. p. 1–34.
-
Bassetti M, Vena A, Croxatto A, Righi E, Guery B. How to manage Pseudomonas aeruginosa infections. Drugs Context. 2018;7:212527.
-
Nagoba B, Davane M, Gandhi R, et al. Treatment of skin and soft tissue infections caused by Pseudomonas aeruginosa—A review of our experiences with citric acid over the past 20 years. Wound Medicine. 2017;19:5–9.
-
Lama IY,Cheung LK,James A, et al. Ecthyma gangrenosum: A case report in a child with acute lymphoblastic leukaemia. JPRAS Open. 2024;40:215-221
-
Muggeo P, Zama D, Decembrino N, et al. Ecthyma gangrenosum in children with cancer: diagnosis at a glance. A retrospective study from the Infection Working Group of the Italian Pediatric Hematology Oncology Association. Pediatr Infect Dis J. 2022;41(3):238–42.
-
Koley S, Das G, Mandal RK, et al. Acute leukemia presenting as primary ecthyma gangrenosum. Indian J Cancer. 2014;51(4):598–9.
-
Ungaro R, Mikulska M. The skin and soft tissue infections in hematological patients. Curr Opin Infect Dis. 2020;33(2):101–9.
-
O’Sullivan GM, Worsnop F, Natkunarajah J. Ecthyma gangrenosum, an important cutaneous infection to recognize in the immunosuppressed patient. Clin Exp Dermatol. 2018;43(1):67–9.
-
Martínez-Longoria CA, Rosales-Solis GM, Ocampo-Garza J, Guerrero-González GA, Ocampo-Candiani J. Ecthyma gangrenosum: a report of eight cases. An Bras Dermatol. 2017;92(5):698-700.
-
Spernovasilis N, Psichogiou M, Poulakou G. Skin manifestations of Pseudomonas aeruginosa infections. Curr Opin Infect Dis. 2021;34(2):72–9.
-
Gałązka P, Kaczor P, Kałużny K, Leis K. Ecthyma gangrenosum as a serious complication of Pseudomonas aeruginosa infection in departments of pediatric oncology. Adv Dermatol Allergol. 2021;38(4):537–43.
-
Natarelli N, Aslam S, Greene JN. Ecthyma gangrenosum of the groin resistant to ciprofloxacin, cefepime, metronidazole, and meropenem—what’s next? Infect Dis Clin Pract. 2025;33(3):e1444.