Analysis of Large Language Models in Clinical Workflow Management, Cost, and Productivity Performance Through Stepwise Uro-Oncology Scenarios
Abstract
Introduction: This study compared the time-based cost-efficiency and generative efficiency of three large language models (LLMs), ChatGPT-5, Gemini 2.5, and Claude Opus 4.1, using comprehensive stepwise uro-oncologic scenarios, and evaluated the effect of optimized prompting on economic performance.
Methodology: Ten clinically realistic stepwise scenarios (five prostate and five bladder cancer cases) were developed by a radiation oncologist, a urologist, and a medical oncologist through expert consensus. Each scenario comprised three stages: diagnosis, treatment, and follow-up, with two open-ended questions per stage. One pilot case tested two prompting strategies: standard stepwise and Sequential Waterfall Prompting (SWP), where preceding steps were cumulatively appended. All scenarios were presented to each model through its APIs using the selected method. For each response, input, reasoning, and output tokens and response times were recorded. Total Cost (USD) was calculated per question using official token pricing. Models were compared for Total Cost, Response Time (s), Generative Efficiency (Output/Input), and Economic Efficiency (Cost/Time). Non-parametric Kruskal–Wallis and one-way ANOVA tests were applied in SPSS v20, comparing both models and scenario steps.
Results: Using SWP, a 17-fold cost reduction was achieved compared with standard stepwise prompting. The average cost per scenario was 0.98 USD, and the average total token usage was 35,124 tokens (input and output combined). Significant differences were found among models for cost, response time, and generative efficiency (p < 0.001). Gemini 2.5 achieved the lowest cost and fastest responses, while ChatGPT-5 demonstrated the highest Generative and Economic Efficiency, reflecting a balanced trade-off between performance and resource utilization. Claude Opus 4.1, despite its higher cost, remained competitive in response speed (Figure 1).
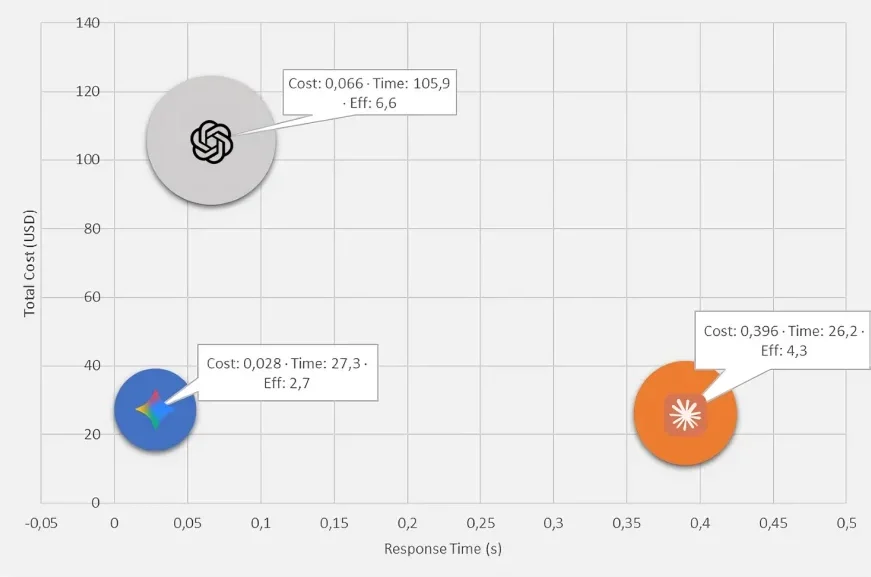
Figure 1: Cost-time-generative efficiency map illustrating the multidimensional relationship among three LLMs
In the stepwise analysis, cost differences were not significant (p = 0.066), but the diagnostic stage showed shorter response times (p = 0.019) and higher generative efficiency (p = 0.021) compared with the treatment and follow-up phases (Table 1).
Table 1: Comparison of three LLMs and clinical steps across cost, time, and efficiency metrics
| Model comparison | Model | Mean ± SD | Mean rank | p-value | Post-hoc comparison |
|---|---|---|---|---|---|
| Total Cost (USD) | GEMINI | 0.0280 ± 0.0068 | 31.05 | < 0.001 | OPUS>GPT>GEMINI |
| GPT | 0.0663 ± 0.0163 | 89.95 | |||
| OPUS | 0.3958 ± 0.0130 | 150.5 | |||
| Response Time (s) | GEMINI | 27.30 ± 6.30 | 64.8 | < 0.001 | GPT>GEMINI≈OPUS |
| GPT | 105.93 ± 42.64 | 150.43 | |||
| OPUS | 26.22 ± 6.43 | 56.27 | |||
| Generative Efficiency (Output/Input) | GEMINI | 2.70 ± 0.96 | 40.8 | < 0.001 | GPT>OPUS>GEMINI |
| GPT | 6.64 ± 1.88 | 139.73 | |||
| OPUS | 4.31 ± 1.43 | 90.97 | |||
| Economic Efficiency (Cost/Time) | GEMINI | 0.00102 ± 0.00067 | 90.13 | < 0.001 | OPUS>GEMINI>GPT |
| GPT | 0.00066 ± 0.00015 | 30.87 | |||
| OPUS | 0.01568 ± 0.00257 | 150.5 | |||
| Step comparison | Step | Mean ± SD | Mean rank | p-value | Post-hoc comparison |
| Total Cost (USD) | Diagnosis | 0.1543 ± 0.1675 | 77.72 | 0.066 | No significant difference |
| Treatment | 0.1675 ± 0.1681 | 96.22 | |||
| Follow-up | 0.1684 ± 0.1650 | 97.57 | |||
| Response Time (s) | Diagnosis | 41.89 ± 28.24 | 75.05 | 0.019 | Treatment≈Follow-up>Diagnosis |
| Treatment | 56.12 ± 43.77 | 98.97 | |||
| Follow-up | 61.44 ± 56.81 | 97.48 | |||
| Generative Efficiency (Output/Input) | Diagnosis | 5.04 ± 2.30 | 102.92 | 0.021 | Diagnosis > Treatment > Follow-up |
| Treatment | 4.59 ± 2.12 | 91.97 | |||
| Follow-up | 4.03 ± 2.04 | 76.62 | |||
| Economic Efficiency (Cost/Time) | Diagnosis | 0.00615 ± 0.00769 | 91.38 | 0.978 | No significant difference |
| Treatment | 0.00547 ± 0.00676 | 89.4 | |||
| Follow-up | 0.00575 ± 0.00713 | 90.72 | |||
| Total Cost (USD) | Diagnosis | 0.1543 ± 0.1675 | 77.72 | 0.066 | No significant difference |
Conclusion: This study offers a preliminary yet objective evaluation of LLM performance within structured clinical workflows and delineates model-specific strengths. Gemini 2.5 emerged as the most cost- and time-efficient model, whereas ChatGPT-5 achieved the most favorable overall performance, characterized by superior Generative and Economic Efficiency. The increased productivity observed during the diagnostic phase likely reflects its more focused analytical demands. These findings underscore that future LLM-based clinical decision-support systems should be optimized not only for cost and speed but also for clinical accuracy and explainability (1–3). Continued validation of clinical accuracy will be essential to determine the real-world applicability of these models in oncologic practice.
Conflict of interests: The authors declare no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
License: © Author(s) 2026. This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, and unrestricted adaptation and reuse, including for commercial purposes, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
To view a copy of this license, visit https://creativecommons.org/licenses/by/4.0/.





